Spheroids - 3D Cell Culture
October 2019
Over the last twenty years, a central focus in drug discovery efforts has been the progressive incorporation of in vitro assays with ever increasing physiological relevance. Industrialized screening of chemical entities for binding to purified protein has been replaced by functional assays involving cells. These cell-based assays have themselves evolved to higher levels of physiological relevance through the use of endogenous expression of drug target receptors and the use of primary cells. Furthermore, the widely practiced tissue culture methods of plating cells in monolayers on labware, are in turn being supplanted by models involving cell aggregates, where the extracellular matrix components, cell-to-cell and cell-to-matrix interactions that are important for typical cell function in vivo are retained in large part. These 3D cell culture methods can be divided into two camps that either use support matrices scaffolds to encourage cells to aggregate or special coatings on labware to prevent plastic to cell adhesion and thus induce cells to self-aggregate. These self-aggregated cells are typically termed spheroids.
In this TekTalk issue, we will demonstrate some applications of the use of spheroids and how BioTek products can enable spheroid assay workflows.
Featured Applications
Long-Term Hepatotoxicity Studies using Cultured Human iPSC-Derived Hepatocytes
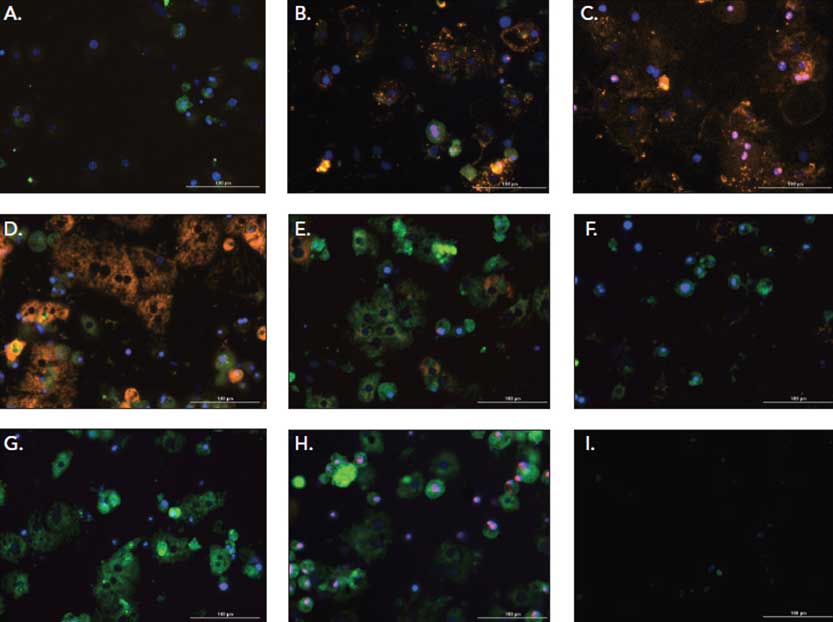
Quantitative microscopy was employed to determine the effect of drugs on human iPSC derived hepatocytes aggregated into spheroids. Three phenotypes of toxicity were measured using fluorescent probes: reactive oxygen species generation, mitochondrial membrane potential decline and plasma membrane rupture. Results were compared to hepatocytes cultured in flat bottom microplates. Spheroid formation enabled much longer kinetic studies, extending out to 14 days. This capability allowed for measurement of full pharmacology and phenotype.
Automated Media Exchange for Spheroid Cultures Using a Novel MultiFlo FX Accessory
Three dimensional (3D) spheroidal cell models have become a mainstay in life science research due to the ability to mimic in vivo-like environments. Performing media exchanges and washes with spheroids in cell repellent microplates can be problematic due to the risk of accidental spheroid removal. By incorporating a novel peristaltic pump-based tool, these procedures can be carried out in a controlled manner that eliminates spheroid disruption and removal, enabling long-term 3D experimental procedures requiring multiple media exchanges.
Product Spotlight
Automated, Gentle Media Exchange
3D cell culture workflows typically require regular media exchanges to ensure cell viability and proliferation. Many methods for media exchanges, spheroid washing and re-dosing can result in accidental spheroid disruption or removal in one or more wells, causing problems with the assay. The new Automated Media Exchange (AMX) module for BioTek's MultiFlo FX automatically removes spent media, replacing it with fresh media, or fresh media containing treatments. The AMX module can be added to existing MultiFlo FX Multi-Mode Dispensers to help your laboratory enable automated media exchanges for your 3D cell culture workflows.
Tek Tips
Get Better Results by Optimizing Your Image Capture Steps
Furthermore, cells in multiple planes, whether floating or living within a large organoid, can cause inconsistency with autofocus methods, bringing into question the reliability and reproducibility of later analysis steps. Autofocus failings can also be prevalent with gel-based scaffolds and other tools frequently used in 3D experiments. So how can you optimize your image capture steps for best results?
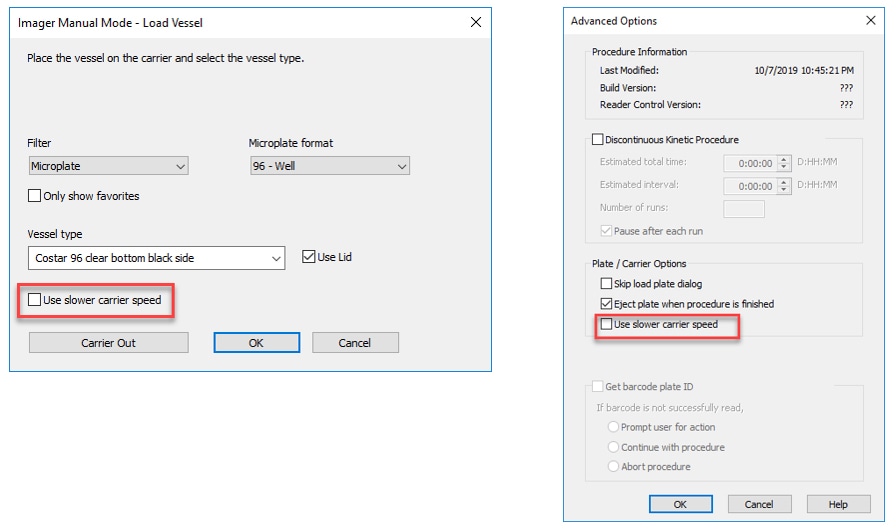
1. Slow the Carrier Speed: New in Gen5 3.08 is the ability to slow the speed with which the carrier enters the imager. This option is available in both Manual Mode and Experiment Mode, and is particularly recommended when imaging suspension or spheroid-based cultures during extended kinetics utilizing the BioSpa 8 Automated Incubator.
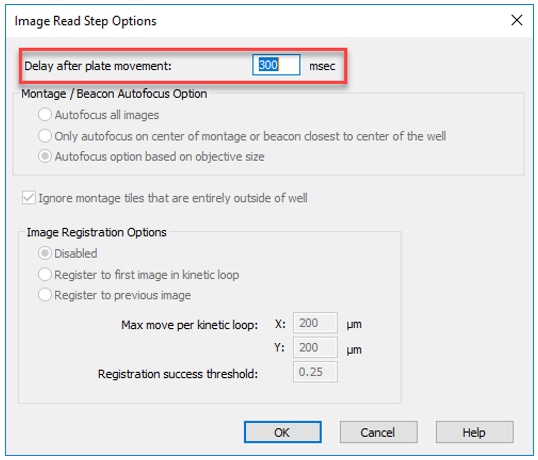
2. Add a Delay After Plate Movement: By default, Gen5 builds in a 300ms delay between carrier movement and image capture. This value can be reduced for faster acquisition OR increased to allow suspension cultures or spheroids to resettle after movement.
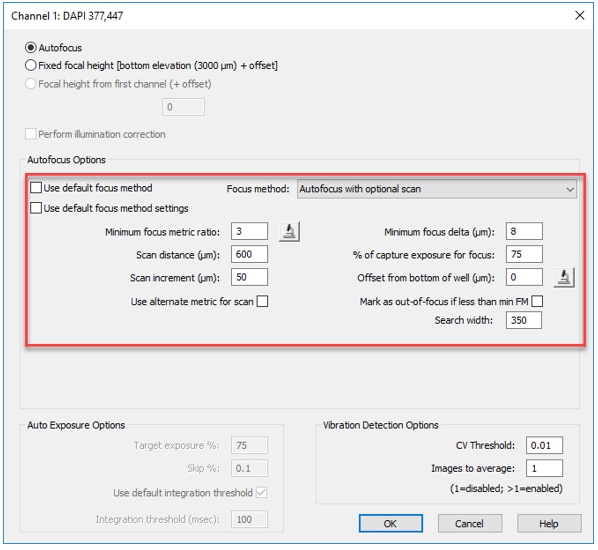
3. Optimize the Autofocus Method: Even for spheroid and suspension cultures, the laser autofocus provides superior speed and performance; however the unique conditions inherent in these cultures sometimes require further optimization. By de-selecting the Default focus method and settings, you gain additional freedom in defining parameters such as the scan distance and increment, or utilizing alternative methods, such as user-trained autofocus. Read more about Autofocus Methods in the Imaging Training Guide that was included with your BioTek Lionheart FX Automated Microscope or Cytation Cell Imaging Multi-Mode Reader.
4. Adjust the Correction Collar: While we're on the topic of focusing, don't forget to increase the correction collar settings on your 20x, 40x, and 60x objectives when working with thicker biology!
5. Call your Field Applications Scientist (FAS): When you're feeling stumped or frustrated or just want to brainstorm ideas to improve your assay (3D or anything else), contact BioTek's FASTeam for scientific support!
Automated Media Exchange of Non-Adherent Cells and Spheroids
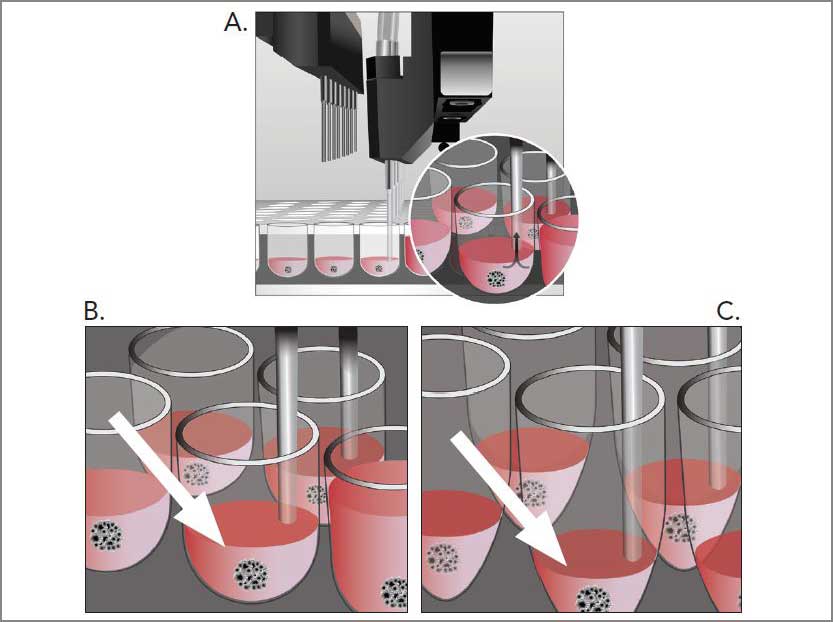
Media exchange is an essential element of successful long-term cell culture in both high and low throughput experiments. Automated Media Exchange streamlines this process by reducing workflow duration, increasing reproducibility and therefore saving time and labor. However, some cell culture systems are sensitive to the disruptive effects of media exchange requiring specialized liquid handling equipment with optimized parameters for successful analysis post exchange. BioTek's patent-pending Automated Media Exchange (AMX) module, available on the MultiFlo FX, is designed to gently and reliably exchange media for biology sensitive to the disruptive forces of fluid movement. The AMX module uses two peristaltic pumps to gently aspirate, and subsequently dispense, media from up to eight wells simultaneously.
Here we demonstrate the ability of the AMX module to gently perform media exchange of spheroids. Importantly, the AMX module is able to exchange media with no discernable displacement of the well biology, as well as complete retention of suspension cell numbers. Technical tips are provided regarding media exchange settings and guidelines for optimization using the MultiFlo FX with AMX.

The MultiFlo FX with AMX module.
3D Spheroid media exchange in 96-well, round bottom ULA plate:
- Define the aspiration height for your vessel
Careful empirical experimentation needs to be performed when working with suspension or non-adherent cells such as the formation of spheroids in ULA plates. The plate dimensions are required to determine a starting point for both aspiration and dispense heights as described above as well as substantially more residual volume in comparison to adherent cells. The composition of the spheroids, and subsequent level of cellular cohesion between cells in the spheroid, will determine the residual volume required. We found the 50% residual volume is a good starting point in a standard 96-well microplate format. For tightly formed, structurally stable spheroids, we found up to ~ 75% of media can be exchanged per cycle. The exact aspiration height required to leave behind a similar volume in your experiment is best determined empirically using your microplate, and your media. - Use “slow carrier mode” feature for washing and imaging of suspension cells
Normal handling including transporting the plate can disturb non-adherent cells. The slow carrier feature available in both Liquid Handling Control (LHC) and Gen5 software provides slow, smooth plate movements throughout the liquid handling and imaging processes. This feature was designed specifically to minimize disruption of sensitive cells and structures. - Determine additional aspiration and dispense parameters
The AMX module takes advantage of two peristaltic pumps: one for aspiration and one for dispense. The speeds can be individually optimized for each step. Begin by using the slowest aspiration and dispense rates and verify that your biology is undisturbed. Here we demonstrate spheroid media exchange using the slowest aspiration and dispense rates. As described above, the aspirate and dispense parameters will need to be optimized for the plate type and biological system. It is recommended to aspirate directly above spheroids when spheroid positioning needs to remain constant; centered in the well of a round bottom, ULA plate in this case. Visualization of this process is depicted in a short video found here: Automated Gentle Media Exchange for Spheroid Assays using the AMX module for MultiFlo FX. - Dispense near the well edge
The position of dispense and aspiration needles within the well is fully adjustable using LHC software. Dispensing media near the well edge allows a liquid bridge to form against the sidewall, and avoids droplets hitting the well media surface. - Minimize the distance between the dispense needle and the final liquid surface
Dispense needle height is fully customizable in LHC software. To avoid cross-contamination between wells, it is important that dispense needles are not submerged below the final media level in the well. Dispensing from far above the surface interface, however, encourages droplet formation and increases mixing forces. The dispense height was optimized to just above the final well volume of 200 mL for this 96-well vessel.
Best practices and tips:
Below is a short list of tips to consider when setting up AMX module media exchange for suspension cells:
- Start with the slowest possible aspiration and dispense settings, and modify as needed
If total washing time is a factor for your experiments, increase dispense or aspiration speeds incrementally and always verify cell retention. - Allow non-adherent cells to settle to the bottom of the well before washing
Ensure cells have had adequate opportunity to settle to the bottom of the vessel before attempting a media exchange to avoid directly aspirating cells near the top of the liquid column. Typically, a media exchange will occur days after initial plating of cells, so most non-adherent cells will be well settled to the bottom of the vessel. Spheroids typically settle to the bottom relatively quickly once formed, on the order of minutes. - Validate your exchange settings using relevant experimental liquids
Although tempting to use less expensive and more abundant liquids such as saline or water; liquids demonstrate different liquid handling characteristics due to differences in physical properties such as surface tension. Therefore, the use of the final experimental liquid is recommended for validating settings, typically complete media. - Leave more media in the well, and run more wash cycles
As a general principle to avoid disturbing the biology at the bottom of the well, minimize the amount of media aspirated per wash cycle, and simply repeat the wash cycle more times to achieve a complete media exchange.
Spheroid - 3D Cell Culture Resources
Application Notes
- Automated Media Exchange for Spheroid Cultures Using a Novel MultiFlo FX Accessory
- 3D Spheroid-Based Tumor Invasion Assay
- Brightfield and Fluorescence Imaging using 3D PrimeSurface® Ultra-Low Attachment Microplates
- An Image-Based Method to Detect and Quantify T Cell Mediated Cytotoxicity of 2D and 3D Target Cell Models
- Long-Term Hepatotoxicity Studies using Cultured Human iPSC-Derived Hepatocytes
Webinar
Biomolecular and Cellular Quantification Using a Multi-Mode Microplate Reader
Presenter: Peter J. Brescia Jr., MSc, MBA, Applications Scientist
Quantification of purified nucleic acids and protein is a preliminary step for a multitude of downstream applications in a wide range of scientific fields of study. Depending on the sample and application, there is a need for a range of methods to best quantify the biomolecule of interest. Here we will discuss the various methods available to accurately and precisely determine a wide range of analyte concentrations typically found during purification. Methods will include the use of micro-volume, microplate and standard one-centimeter path length vessels using a range of detection methods available in microplate readers.
For Research Use Only. Not for use in diagnostic procedures.