Automated Imaging with Water Immersion Objectives
January 2024
Fluorescence light microscopy is a powerful tool that provides spatial insight into biological processes. Water immersion objectives capture more light than air objectives, a trait that is especially useful in imaging workflows where the sample is typically contained within an aqueous solution and can be two-dimensional (2D) or three-dimensional (3D). This enhanced light capture property leads to several key advantages and benefits that overcome the challenges of imaging 3D and other thick samples.
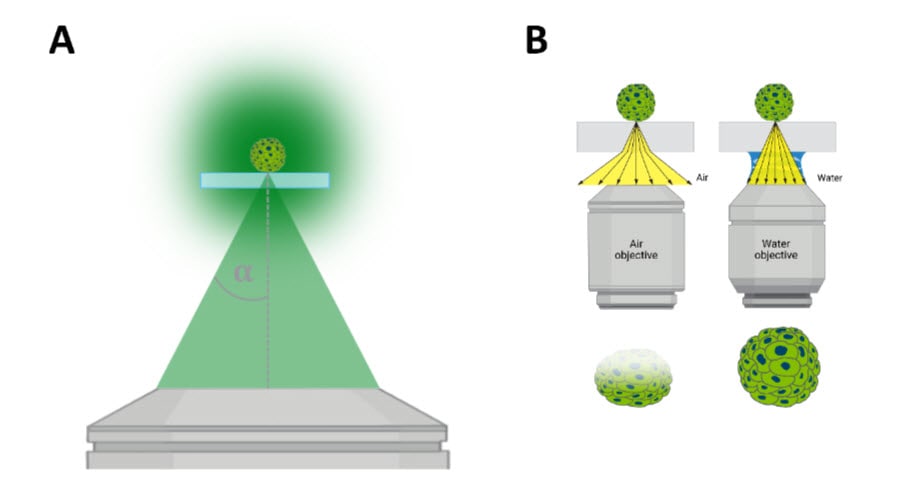
Image quality (z-resolution and contrast) is affected by two major factors (Figure 1): 1) numerical aperture (NA) and refractive index (n). The numerical aperture (NA) is a measure of how much light a microscope objective can collect and is influenced by the half-aperture angle (α) (Figure 1A). A higher NA objective can collect more light compared to an objective with a relatively lower NA. In practice, this allows water immersion objectives to collect equivalent signals (photons) with fewer exposure times, which in turn reduces destructive sample photobleaching and improves signal detection. This also means that they are ideal for collecting sufficient, quantifiable signal from dim samples that would otherwise require prolonged exposure times. Additionally, the intensity of emitted light decays with imaging depth, and thus light detection is dramatically reduced at deeper z-planes.
Image quality is also negatively impacted by transitions in the medium that emission light must traverse when returning from the sample to the camera used for signal detection. The refractive index (n) is a measure of how the speed of light is influenced as it passes through a material, such as the media that contains a thick sample to be imaged. Within an imaging system, matching the refractive index between the sample, mounting medium, and optical interface minimizes diffracted light, which in turn improves image quality (Figure 1B).
In this edition of TekTalk we’ve compiled diverse resources that describe the advantages of water immersion objectives and practical applications, as well as general tips and best practices for optimizing results.
Featured Application Note

Advancements in Automated Confocal Imaging
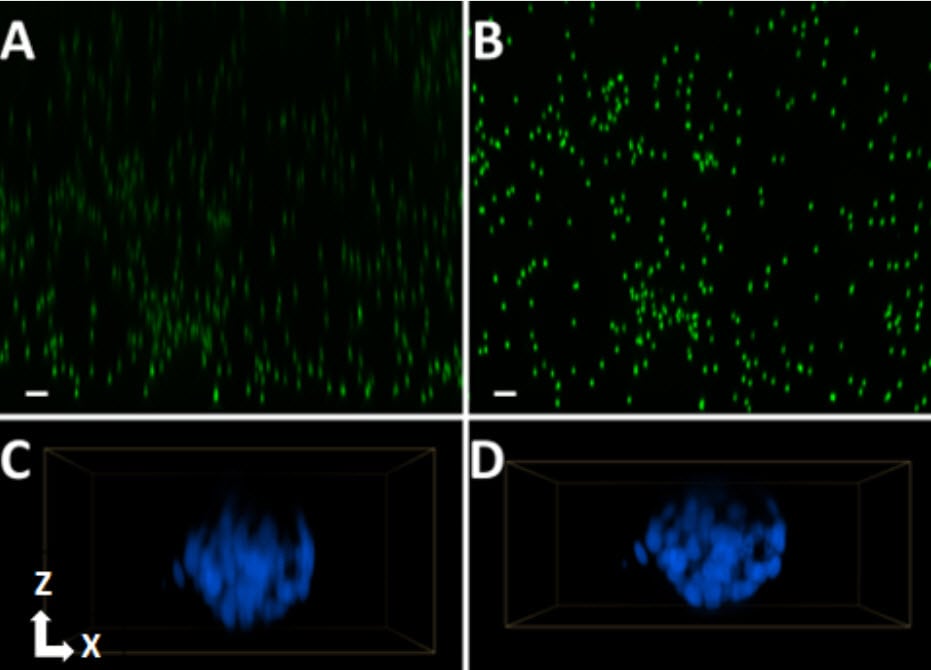
Light microscopy is a powerful tool that provides spatial insight into biological processes. Confocal microscopy improves image quality (e.g., Z-resolution and contrast) by reducing out-of-focus light. Water immersion images with high numerical apertures further improve image quality by collecting more light (relative to air objectives with lower numerical apertures but equal magnification), and better correcting for light distortion associated with refractive index mismatches between aqueous samples and the optical path of a microscope. This application note demonstrates the utility of the Agilent BioTek Cytation C10 confocal imager reader in a nuclear translocation assay. In this assay, TGF-β1-stimulated SMAD2/3 phosphorylation and nuclear translocation are quantified in both 2D adherent and 3D spheroid cultures of A549 adenocarcinoma cells. The new water immersion capabilities of the Cytation C10 allow for more efficient fluorescent signal detection of this low abundance phosphorylated SMAD2/3 species, improved Z-resolution of spheroids, and compatibility with high-throughput applications, such as multi-replicate dose response curves.
Tek Tips
Ensure your sample is in an aqueous solution
The improvements to image quality achieved with a water immersion system is not only because there is more efficient light collection with a higher NA objective, but also because the refractive index is better matched among the optical path when imaging an aqueous sample. Variations in refractive indices will degrade/distort the resultant sample image. By immersing your sample in an aqueous solution while imaging, such as culture media or PBS, you will ensure that the refractive index is matched, and sample image quality is optimal.
Ensure your sample is as close to the coverslip or well bottom as possible
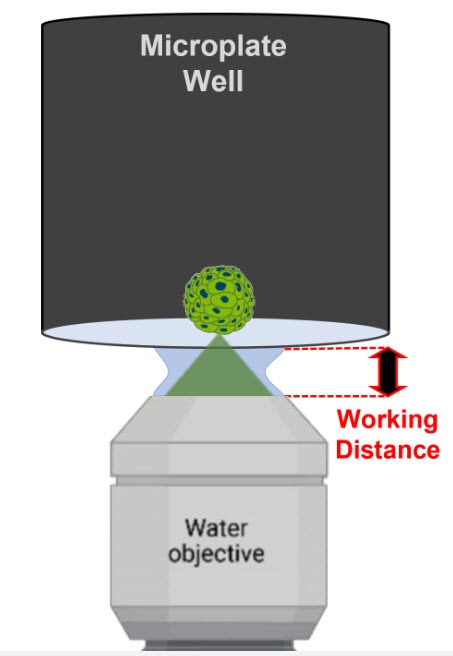
Water immersion objectives are well suited for thin samples, such as adherent cells or histological sections, because these objectives tend to have a shorter working distance. For example, the working distance of the 60X water immersion objective compatible with the Cytation C10 is 280 µm, whereas its air counterpart has a working distance of up to 2.2 mm. However, it is still practical to image thicker samples such as spheroids, organoids, or superficial regions of tissue. To successfully image these types of samples with a short working distance objective, ensure that the sample is as close to the imaging interface (e.g., coverslip or well bottom) as possible.
Water Immersion Technology
Discover the unique technology and hardware features supporting the Cytation C10 automated water immersion system.
Webinar
Achieving deeper insights into complex cellular models through optical enhancements to automated confocal imaging
Wednesday, January 24, 2024 | 12 PM EDT
Presenter: Ernest Heimsath, Ph.D. - Application Development Scientist; Agilent Cell Analysis Division
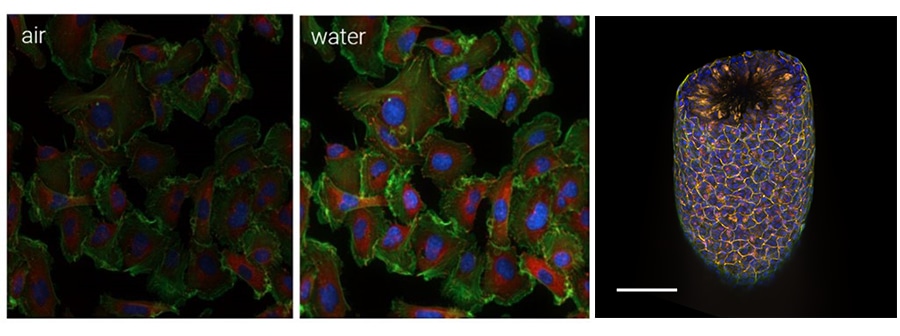
Confocal light microscopy is a powerful tool that, by reducing out-of-focus light, provides three-dimensional insight into biological processes. Combined with high numerical aperture water immersion objectives, image quality is further improved by more efficient light collection and correcting for light distortion associated with refractive index mismatches.
In this webinar, we introduce two new features of the Agilent BioTek Cytation C10 confocal imaging reader: water immersion imaging, which allows for more efficient, gentle detection of dim fluorescent signals, improved z-resolution, and compatibility with high-throughput applications in microplate formats, and the 60 µm deep-sectioning spinning disk (DSD), which reduces pinhole crosstalk, a phenomenon that results in high background signal when imaging thick biological samples. We will provide a brief overview of the optical challenges that these improvements overcome, then present applications that benefit from their implementation. These new features of the Cytation C10 confocal imaging reader deliver several key advantages and benefits while overcoming critical challenges of confocal imaging.
This presentation will include:
Featured Product
Cytation C10 Confocal Imaging Reader
The Cytation C10 confocal imaging reader combines automated confocal and widefield microscopy with conventional multimode microplate reading. The automated water-immersion objectives capture more light, which can lead to lower exposure times and reduced phototoxic impacts on live cells. A proprietary bubble detection function prevents blurring or other image degradation caused by an inconsistent water column during water-immersion imaging. The spinning-disk confocal module and accompanying disk pinhole options allow a researcher to look deeper into thick biological samples with improved clarity and detail. The Cytation C10 also includes widefield fluorescence, brightfield, and phase-contrast optics. The multimode module has monochromator-based optics that incorporate variable bandwidths for specificity and sensitivity. System control, image, and data analysis are provided by the Agilent BioTek Gen5 software.

