In Vitro Cell Migration Assays
March 2024
Cell migration is a fundamental process in the development and maintenance of multicellular organisms. It is essential for tissue formation during embryonic development, wound healing, and immune responses. Cells migrate in response to various external cues, including chemical, mechanical, and light. The process of cell migration is complex and involves a coordinated interplay of many different cellular components.
There is also considerable interest in understanding the molecular mechanisms of cell migration across broad life science research fields, due to the recognition that cell migration plays a critical role in many diseases, including cancer, cardiovascular disease, and neurodegenerative disorders. A better understanding of cell migration could lead to the development of new therapeutic strategies for these diseases.
There are several technical challenges associated with studying cell migration. One notable challenge is that while cells migrate within their native environment in three dimensions, most assays are performed in two dimensions, often on rigid substrates that are orders of magnitude stiffer than the regimes cells typically engage within tissue. Additionally, cells migrate in response to a variety of different cues, and it can be difficult to isolate the effects of a single cue. However, well-controlled 2D cell migration assay formats can effectively quantify relative cell migration behavior and tease out driving factors. Furthermore, recent advancements with 3D models and cell imaging techniques, and image analysis tools, are adding additional relevance and insights for cell migration studies.
In this issue of TekTalk, we’ve compiled diverse resources describing automated imaging-based solutions for conducting in vitro cell migration studies, including conventional Boyden chamber formats, fully automated scratch wound healing assays, and high throughput cell exclusion zone formats with automated kinetic analysis. Featured application notes provide examples of expected results and detailed instructions on setting up the assays. Additionally, TekTips are provided to describe approaches for increasing throughput with automation solutions, as well as guidance on optimizing conditions and analysis to achieve accurate results.
Featured Applications

Automated Imaging and Analysis of 2D Chemotaxis
The ability of cells to migrate is a fundamental biological process in development and immunological responses. It is also the defining feature of metastatic cancers. The process of cellular motility towards external cues is collectively directional cell migration, for which migration towards a soluble chemical stimulant is referred to as chemotaxis. Measuring chemotaxis requires the ability to track cellular movement accurately and reliably.

Incorporation of a Novel, Automated Scratch Tool and Kinetic Label-Free Imaging to Perform Wound Healing Assays
The wound healing or "scratch" assay is one of the most highly used in vitro methods to monitor and quantify collective cell migration. The current standard involves manual wound creation, which yields low reproducibility between wounds, high variability within generated data, and possible false conclusions regarding test molecules. Using an automated wound creation tool, in addition to kinetic image capture and analysis, repeatable wounds and robust and repeatable results are easily attained.

Automated Label-Free Method for Measuring Cell Migration in Real-Time with the Oris Pro Assay
Cell migration depends on complex environmental signaling events and induced structural changes to the cytoskeleton. Multicellular organisms rely on cell migration for a vast array of biological processes, including embryonic development, immune responses wound healing, and cancer metastasis. This application note demonstrates an automated kinetic imaging-based approach to investigating cell migration. This convenient label-free method enables robust analysis of cell migration in 96- or 384-well microplates for high-throughput applications.

Performance of a Label-Free Image-Based 2D Scratch Wound Healing Assay to Monitor Cell Migration and its Inhibition
In this application note experiments were performed to track the ability of various cell types to move into a created gap or heal the wound. In addition, inhibition of wound healing with Cytochalasin D is also shown. Culture-Inserts and 35 mm µ-Dishes, provided by ibidi, were incorporated to perform the wound healing experiment process. Wound closure was monitored using an Agilent BioTek Lionheart FX automated microscope, and cellular analysis of the kinetic images captured was automatically performed using Agilent BioTek Gen5 microplate reader and imager software.
Tek Tips
Optimize microplate migration assays with automated wash procedures for cleaner and more reproducible results
Conducting wash procedures after scratches are created, or cell exclusion zones are formed, increases cell migration assay reliability and reproducibility (see featured video below).
Washing can be done manually using a multi-channel pipette
- Remove ~80% of well volume
- Replace with fresh medium by dispensing down the side of the well
- Repeat 2 times
Alternatively, an automated liquid handler (e.g. ELx50, 405 TS, MultiFlo FX) can be used to increase the efficiency and effectiveness of post-scratch washes

Selecting the most appropriate cell migration assay format
Available instrumentation
Automated live-cell imaging systems provide a flexible instrument platform to support a broad range of assay formats, including both endpoint and kinetic. Cell migration assays designed for use with microplate readers offer a straightforward approach but rely on fluorescent labels and provide less detail, typically with an endpoint readout.
Research question: Different assays measure different aspects of cell movement
- Cell migration, invasion, or both? For invasion assays, coat the transwell membrane, or overlay the cell culture and wound area for scratch wound healing assays, with a biologically relevant extracellular matrix (ECM) or hydrogel. The matrix gel acts as a barrier for cells during invasion. For migration assays, omit the ECM coating step unless appropriate to coat the cell substrate prior to cell seeding to enable desired attachment characteristics.
- Individual cell movement or collective cell migration? Measuring the movement of individual cells can provide detailed insight into cell migration behavior, particularly within the context of chemotaxis assays. Characterizing migratory behavior of individual cells within a 2D environment typically requires object tracking software to effectively and efficiently quantify cell movement. Alternatively, collective cell migration, in which a cell culture front is measured as it advances into a neighboring area, is often used in the context of embryogenesis and wound repair.
Cell Type and Pore Size:
- Boyden chamber (transwell) style migration assay formats are available with membranes consisting of a range of pore sizes to address varying cell types and characteristics.
- Ensure that the pore size matches your cell dimensions to prevent cell clogging or unregulated cell movement.
- Refer to the product guidelines and primary literature to determine the ideal pore size for each given cell type.
Remember that each assay format has its advantages and limitations, and understanding the nuances of each within the context of your biological system will help you choose the right format for your specific research needs.
Webinar
Navigating the cell migration assay landscape: How to bring together the most appropriate tools and approaches to ensure robust, accurate results
March 20, 2024 | 12 PM EDT
Presenter: Joseph E. Clayton, Ph.D., Global Scientific Program Manager, Agilent
Cell migration plays a pivotal role in various biological processes, from embryonic development to wound healing and cancer metastasis. In this Agilent Cell Analysis webinar, we will provide a review of existing assay formats for studying cell migration behavior, including the scratch wound healing assay, barrier cell exclusion formats (e.g., Oris and Ibidi), chemotaxis assays, and 3D cell culture formats for characterizing cell invasion. We’ll provide guidance on selecting the most appropriate assay format considering several factors, such as biological context/sample type, throughput, cost, and desired readout. Additionally, we will provide detailed recommendations for optimizing image-based cell migration assays within microplate formats, with a focus on:
- Increasing reproducibility
- Accurate quantification using automated image analysis tools
- Microenvironment (e.g., co cultures and extracellular matrix)
- Fluorescence vs label-free approaches
- Kinetic live-cell imaging
Featured Videos
Optimizing Cell Migration Scratch Assays with Effective Post-Scratch Microplate Washing

Featured Products
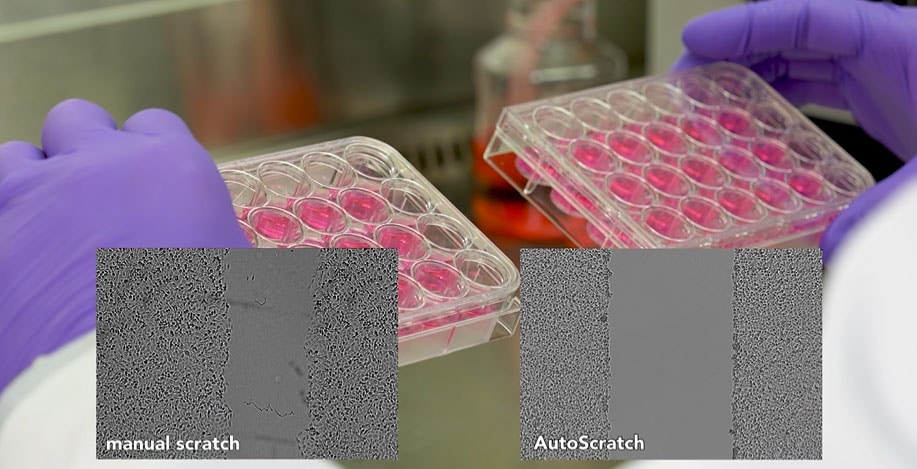
Agilent BioTek AutoScratch and Scratch Assay app
The Agilent BioTek AutoScratch Wound Making Tool automatically creates reproducible scratch wounds in cell monolayers grown in 24- or 96-well microplates for cell migration and invasion studies. AutoScratch automates the sample prep for imaging using BioTek’s Cytation Cell Imaging and Multi-Mode Readers and Lionheart Automated Microscopes.

Agilent BioTek Lionheart FX Automated Microscope
Lionheart FX Automated Microscope offers up to 100x magnification with brightfield, color brightfield, phase contrast and fluorescence channels for fixed and live cell applications. Optional incubation to 40 °C, CO2/O2 control, and a humidity chamber optimize live cell assays. Gen5 software makes cell image capture, processing, analysis, and publication-ready data and images quick and effortless.

Agilent BioTek Cytation 5 Cell Imaging Multimode Reader
BioTek Cytation 5 is a modular, upgradable multimode reader that combines automated digital microscopy and conventional microplate detection. Cytation 5 includes filter- and monochromator-based detection; the microscopy module provides up to 60x magnification in fluorescence, brightfield, color brightfield, and phase contrast. Incubation to 65 °C, shaking and Gen5 software are standard.
Resources
Application Notes
- Advanced Wound Healing Assay Workflow using Automated Scratch Wound Creation, High Contrast Brightfield and Fluorescence Kinetic Imaging
- Incorporation of a Novel, Automated Scratch Tool and Kinetic Label-Free Imaging to Perform Wound Healing Assays
- Imaging and Analysis of a High-Density Cell Migration Assay
- A Novel High Throughput-Compatible Cell Migration Screening Assay