Immune Cell-Mediated Cytotoxicity
September 2020
As part of the adaptive immune system, specialized lymphocytes, including cytotoxic T lymphocytes (CTL) and NK cells, mount targeted attacks to rid the body of intracellular pathogens and cancerous cells. This immune cell-mediated cytotoxicity can be antibody-dependent or independent and is carried out through induced apoptosis or secretion of cytotoxic granules.
The most popular in vitro method to monitor immune cell-mediated cytotoxicity on target cells is the cell-mediated cytotoxicity (CMC) assay, in which immune effector cells and target cells are added to a microplate as a co-culture along with fluorescent markers for apoptosis or cytotoxicity. Through the incorporation of automated microscopic imaging and cellular analysis, sensitive detection of induced cytotoxicity, as well as visualization of the interplay between effector and target cells, can be achieved. Optimized workflows for both 2D and 3D CMC assay format using the Lionheart FX Automated Microscope and the BioSpa Live Cell Analysis System are described in the resources below.
Featured Applications
An Image-Based Method to Detect and Quantify T Cell-Mediated Cytotoxicity of 2D and 3D Target Cell Models

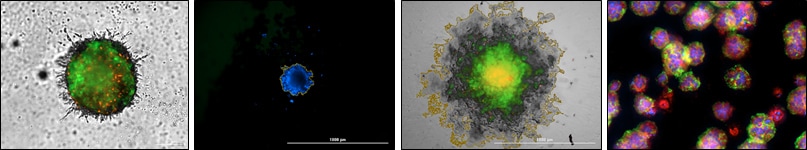
T cell-mediated cytotoxicity plays an important role in a suite of new methods being developed with the goal of boosting a patient's immune system to combat cancer. In order to evaluate and optimize adoptive T cell immunotherapies, sensitive in vitro methods must be included in the testing process. In the procedure described here, phenotypic and quantitative assessments of 2D and 3D target cell necrotic induction were made using automated live cell imaging. It was found that direct activation of T cells produced a significantly greater cytotoxic effect than general activation suggesting that T cells can be "taught" to target and destroy specific target cells.
Validation of an Image-Based 3D Natural Killer Cell-Mediated Cytotoxicity Assay

An in vitro model for Natural Killer (NK) cell-mediated cytotoxicity was developed using a collagen-based scaffold 3D cell culture technology. This technology induced HCT116 cells to aggregate into tumoroids over time which became the target cells during the cytotoxicity assay. Cytotoxicity was assessed by both phosphatidyl serine exposure (apoptosis) and plasma membrane rupture (necrosis) using fully automated workflows. Cytotoxicity was quantified using NK cells alone and with IL-2 stimulation, where a significant increase of cytotoxicity was evident. Cytotoxicity with this model was compared to HCT116 cells adhered to microplates in a conventional 2D format. The 3D model was far superior in both maintaining cell health over time and accurately depicting cytotoxic events.
Product Spotlight
BioSpa Live Cell Analysis System
The BioSpa Live Cell Analysis System offers unique benefits for a wide variety of live cell imaging applications. You can easily capture detailed cellular images and get in-depth cell-level analyses for migration and wound healing assays, 3D spheroid formation and many other kinetic applications in up to 8 microplates or other labware. Built-in scheduling, temperature control, CO2/O2 control and humidity monitoring provide the ideal environment for live cells, allowing you to walk away with confidence, and enables multiple users to run processes without disruption. With up to 60x magnification, patented laser autofocus and advanced cell-level analysis including nuclear and cytoplasm masking define critical, information-rich regions of interest, the BioSpa Live Cell Analysis System offers efficient, powerful automation for your live cell workflows. Add a BioTek washer or dispenser to the system for convenient sample prep and maintenance.
Tek Tips
Automated Imaging of 3D Model Systems
Best Practices to Achieve Consistent 3D Culture for Imaging
The culture of mammalian cells in 3D conditions achieves a more physiological environment and more closely mimics in vivo cell circumstances compared to 2D culture on tissue culture plasticware. 3D culture model systems more closely emulate proper stiffness, nutrient and gas diffusion, cell polarity/morphology, gene expression and proliferation- all important factors that influence research results.
Growth as spheroids or multi-cellular aggregates (MCAs) can be achieved through scaffold, generally containing extra-cellular matrix components, or scaffold-free methods involving forced floating through repulsive polymer coated dishes or physical movement to prevent attachment.
Depending on your plating method and goals for your experiments, the following culture and imaging tips may assist in achieving consistent reproducible results.
Plating in substrates (such as matrigel):
- Level the plate when adding the substrate to ensure even spread. If solidifying substrate under incubated conditions, level the incubator shelf it will rest on as well.
- Centrifuging multi-well plates before or after addition of cells (based on cell type, substrate type and goals) may center substrate or cells evenly.
- With hydrogel matrices, lay 90% of total volume and allow to gel before adding final 10%. This results in a more consistent gel layer and improved uniformity in 96-well or 384-well plate formats.
- Consider specialized 3D culture methods.
- Real Architecture for 3D Tissue (RAFT) from TAP Biosystems provides a specialized system for consistent culture in collagen.
Plating in scaffold-free conditions:
- If utilizing ultra-low attachments plates, round-bottom wells are preferred for imaging and analysis consistency. This well shape forces cells to a uniform central position resulting in consistency in spheroid/ MCA from well to well. This may also aid in imaging as MCAs are not spaced out or clustered against well walls.
- Centrifugation of multiwell plates can also assist in clustering cells uniformly after plating.
- Consider specialized 3D culture methods.
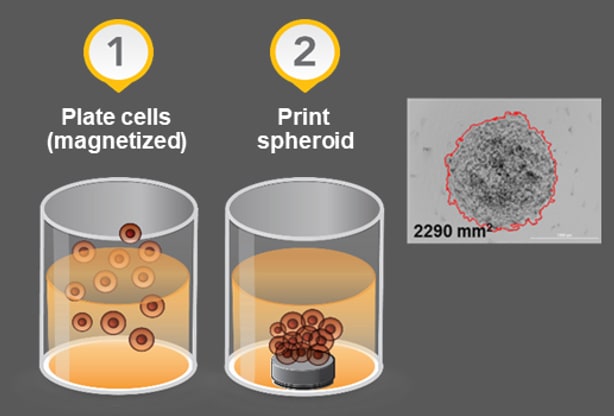
- Target cell magnetization and bioprinting process using n3D technology provides a system by which cells are magnetized and then drawn together using magnets. This is another method to achieve consistent cell clusters and could aid with cell types less prone to sphere formation.
Imaging considerations:
- Automated instruments such as BioTek's Lionheart and Cytation imagers allow selection of low carrier speed to provide slow intake of vessel and minimal physical disruption to 3D cultured cells.
- Increasing the default delay time prior to image capture can provide time for moving cells or liquid in wells to halt movement before image capture. BioTek instruments allow user-determined delays after stage movement.
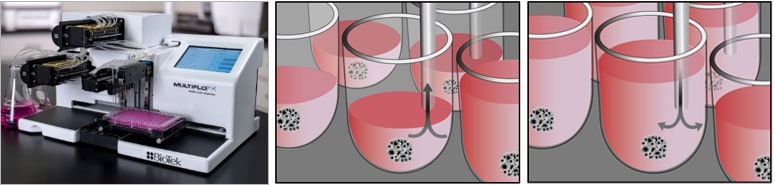
- For long-term culture requiring media exchange, consider automated media exchanges to minimize disruption of MCA/spheroid and provide reliable volume dispensing. BioTek offers the MultiFlo FX AMX module for gentle, controlled aspiration and dispensing rates optimized to prevent spheroid removal in 96- and 384-well plates.
Displayed above is the the MultiFlo FX AMX module and schematics of media removal and addition using autoclavable peristaltic pump cassettes with eight stainless steel tube aspirate and dispense heads.
- Imaging in multiple planes may or may not be required to collect proper data for your experiment. We tend to assume that z-stack imaging is necessary when 3D culture is used, it is possible to collect information critical to your experimental question by capturing images in a single plane. If multiple planes are required, consider time added to image capture and execute several trial experiments to determine the best protocol for multi-plane image capture.
- Acquiring larger steps (spacing with greater micron distance) will cover a broader depth and capture more information moving through a sphere.
- Image capture in a small z-stack could provide information in a more localized fashion within a spheroid without acquiring the whole sphere.
Consider what information is lost/gained by imaging at top or bottom of a sphere vs imaging in the core of a sphere (ex. cell-ECM interactions, anoikis, hypoxia, etc.).
When imaging spheroids in round bottom multi-well plates, try using a fix focal height in your automated image capture procedure. Autofocus metrics may encounter difficulties with round bottom plates. Setting a fixed height at the lowest point in a sphere and z-stacking upwards can provide increased speed of capture and accuracy.
T cell Activation
CD3+CD8+ cytotoxic T lymphocytes (CTL) responsible for T cell-mediated cytotoxicity act by cell-to-cell contact either by releasing granzymes and perforin or through Fas ligand mediated toxicity. As part of the adaptive immune system, these cells mount targeted attacks to rid the body of a variety of compromised cells. One of the most popular in vitro method to monitor CTL effect on target cells is the cell-mediated cytotoxicity (CMC) assay where T cells and target cells are added to a microplate well as a co-culture. The assay relies on proper activation of T cells to elicit the appropriate response. T cells are activated using either general or directed activation methods. General activation includes the use of IL-2 Superkine (Fc), anti-CD3 mAb and anti-CD28 mAb in the culturing media over an extended period, up to 6 days. Directed activation follows the above procedure as well as containing target cells. The directed activation procedure is performed over six days as well and serves to not only activate the T cells, but also teaches them to recognize target cell antigens, allowing for targeted cytotoxicity.
Several method and formats exist to achieve either general or directed T cell activation. General activation is typically performed as a batch process in a standard tissue culture flask. Directed activation is a bit more complex as it requires separation of T cells from the target cells, post-activation. One method of performing the separation is to use ferric particles, such as NanoShuttlePL, which is taken up by target cells and can be used to help form target cell spheroids as well as separate T cells from target cells, post-activation. Additionally, both methods require a media exchange after several days given the requirement for an extended activation period. The major difference between the methods is the co-culturing of T cells and target cells in the directed method concurrent with the use of activating molecules. The process of removing spent media and replacing with fresh media, antibodies, and superkine can be greatly facilitated by using automated liquid handling instrumentation such as the MultiFlo FX with AMX module. Once activated the T cells are removed and transferred to a conical tube for manipulation, such as staining with a CellTracker Dye, allowing for differentiation from the target cells during the cytotoxicity experiment.