3D Cell Culture
October 2018

3D cell culture methods are devised to provide greater physiological relevance to in vitro cell models compared to standard tissue culture where cells are seeded as monolayers on labware. As the name suggests, these models contain a biological thickness of layered cells that provide increased cell-to-cell and cell-to-extracellular matrix interactions that better mimics living tissue.
There are two general groups of 3D cell culture methods: those that use some form of scaffold to build layers of cells; or those that prevent adhesion to labware such that cells naturally self-aggregate. Here at BioTek Instruments, our in-house Applications Lab and Field-based Applications Scientists have used a wide variety of 3D methods suitable for oncology and toxicology research. This issue of TekTalk will be focused on these 3D cell culture methods. This periodical will provide links to our many scientific articles on 3D cell culture including application and technical notes, white papers and webinars; provide information on tips and tricks to enable 3D cell culture assays; and also highlight what some of our customers are doing with our products in 3D cell culture.
Peter Banks Ph.D., Scientific Director, BioTek Instruments
Featured Applications
3D Spheroid-Based Tumor Invasion Assay
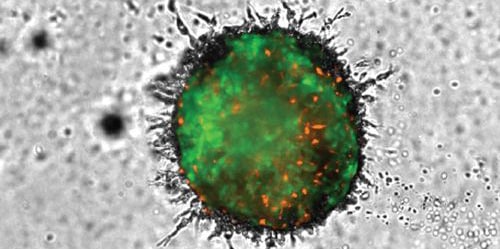
Establishment of in vitro models that mimic tumor invasion as part of the metastatic process, are a critical part of oncology research. The need to incorporate multiple cell types, long-term kinetic analysis, methods to allow 3D invasion into the surrounding matrix, and appropriate detection and analysis, has made it difficult to create new models. The procedure described here meets these needs through inclusion of advanced imaging capabilities allowing for capture of multiple images throughout a range of z-heights, using brightfield and fluorescence channels in a kinetic fashion. Final processed images, following stitching and z-projection, enable accurate cellular analysis to discern the invasive capabilities of 3D cellular structures over time.
3D Cell Culture: A Review of Current Techniques
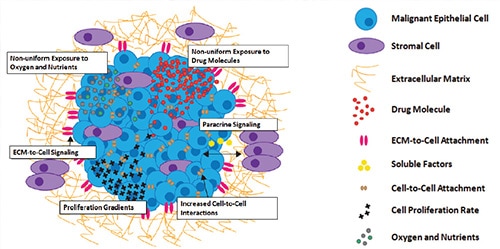
Over the last decade, a central focus of drug discovery efforts has been the incorporation of in vitro testing models that better mimic in vivo conditions found within the target patient. An initial step saw a move away from biochemical assays using purified drug target, in favor of a cell-based approach which utilized over-expression of drug target in common host cell lines, such as CHO and HEK-293. The quest for greater physiological relevance proceeded to the use of primary cells, preferably human if supply was adequate, and the reliance on endogenous expression of drug target should detection technology be sensitive enough. A large percentage of these cell types, being naturally adherent, allowed simple culturing workflows that seeded cells in a coated microplate well, incubating the microplate to encourage the cells to attach in a two dimensional (2D) monolayer before performing the prescribed assay. While providing initial improvements over biochemical and immortalized cell lines, an abundance of evidence now supports the reality that culturing cells in this 2D manner is often problematic and is a relatively poor model for in vivo conditions and behaviors.
Product Spotlight
New! AMX Automated Media Exchange for MultiFlo FX
One of the challenges of working with 3D spheroidal cell models is the need for very gentle media exchange to maintain cell viability for experiments progressing over days or weeks. Manual methods are often cumbersome, and can cause spheroids to be accidentally removed. BioTek's new AMX module for MultiFlo FX Multi-Mode Dispenser gently, automatically removes and dispenses fresh media or fresh media containing treatment concentrations for re-dosing.
Learn more about this novel, patent-pending approach to media exchange for 3D spheroids, tumoroids and other non-adherent structures.
Tek Tips
Incorporating Z-Stacking and Montaging to Image 3D Cell Models
Imaging 3D cell models can present certain challenges when compared to imaging cells plated in a 2D monolayer at the bottom of the well. If assays are performed by hand, involving manual aspiration and dispense steps, spheroids can be moved away from their original position, causing them to be in multiple locations within the well. The same is true for invasion models, where invadopodia and cells expand out into the surrounding matrix. Experiments are also run where plate wells contain multiple 3D structures, such as organoid research. Each of these situations creates the need to capture images not only at multiple z-heights to see cells and other parts of the structure existing on different z-planes, but also in more than one location in the x- and y-axis. The z-stack/montage capability within the Gen5 software allows for easy creation of such a process. Here are some considerations when creating the procedure.
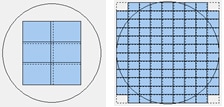
1. The number of images to include in the montage (in rows and columns) should be large enough such that all potential locations of the 3D structure, or structures, are within the captured image area. If a well of a 96-well plate contains a single unattached spheroid or invading tumoroid, a smaller montage may be necessary that covers a portion of the well (below left image). However if multiple organoids are randomly scattered across the well, a large montage may be necessary that covers the entire well (below right image).
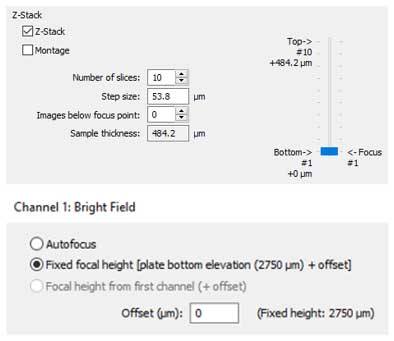
2. For optimization of the total height to capture in the z-axis, manual imaging of positive and negative control wells should be performed. Selecting a range in z-heights that place cells at the interior and exterior of the imaged structure in focus will provide the proper sample thickness. By dividing this value by the step size, or depth of field for the selected objective, the number of slices is determined (below first image).
3. Finally, as multiple images will be captured using the z-stack/montage process per well, imaging speed becomes important. To create the most efficient imaging step possible, selecting a fixed focal height to begin the imaging process for the particular imaging channel per well is recommended (above second image). By previously optimizing the imaging bottom elevation for the selected microplate, all in focus information for the 3D structures of interest will be at that z-height or above. This allows for selection of no images below focus point in the imaging step (above left image) and eliminates autofocusing, allowing for rapid image capture.
Setting up a Z-Stack in Gen5
When setting up a z-stack in Gen5, you can both control the step size between images and the position of the autofocused image in the z-stack. A larger step size is great for a field of spheroids floating in media or embedded in a hydrogel, as you can z-project these into a single, analyzable image to evaluate mean spheroid area or total spheroid number. A smaller step size will give you more control within a single spheroid, for example to better capture the hollow center of an acinar structure or the full breadth of invasive structures. By additionally optimizing the position of the autofocused image within the z-stack, you are able to capture focused events throughout the surrounding environment for more meaningful analysis. If you capture too much, just exclude the extra slices when making your z-projection.
Using the Automated AMX Media Exchange Module and MultiFlo FX to Perform Media Exchanges with Unattached Spheroids
Performing media exchanges with unattached 3D cell models can be difficult and time consuming, and many times leads to high percentages of spheroid loss during the process. Incorporation of the MultiFlo FX and new AMX Media Exchange Module creates an easy to use, reliable media exchange process which prevents spheroid removal. To properly use the module, here are a few tips for optimization.
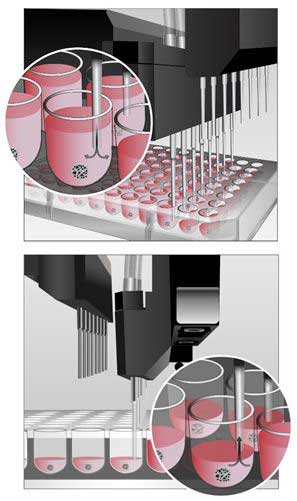
1. For aspiration in 96- and 384-well plates, the location of the aspirate tubes should be placed away from the middle of the well towards the back, right corner. This will also mean that the bottom of the tubes are slightly elevated off the well bottom. Well depths and diameters vary from vendor to vendor. Therefore x-, y-, and z-axis offsets should be checked when using a new plate not previously validated with the module.
2. For dispense in 96-well plates, the location of the dispense tubes should be placed in the same location as for aspiration. When working with 384-well plates, dispense tubes can be positioned in the middle of the well. Due to the smaller diameter and shape of the well bottom, dispensing at this location will not dislodge the spheroid from its current location.
3. Aspirate and dispense speeds should also be optimized. Smaller spheroids created from fewer cells have less mass and can therefore be more easily pulled during aspiration or pushed away from the well bottom during dispense. Therefore slower aspirate and dispense rates should be incorporated here. Spheroids created from larger cell numbers have a greater mass and are more difficult to dislodge from the well bottom. Higher aspiration and dispense rates can be used in these situations.
3D Cell Culture Resources
Application Notes
- Automated Media Exchange for Spheroid Cultures Using a Novel MultiFlo FX Accessory (BioSpa 8, Cytation 5, MultiFlo FX)
- An Image-Based Method to Detect and Quantify T Cell Mediated Cytotoxicity of 2D and 3D Target Cell Models (BioSpa 8, BioSpa System , Cytation 1, Cytation 5)
- Long-Term Hepatotoxicity Studies using Cultured Human iPSC-Derived Hepatocytes (Cytation 5)
- An Automated Walk-Away System to Perform Differentiation of 3D Mesenchymal Stem Cell Spheroids (BioSpa 8, Cytation 1, Cytation 5, EL406, Gen5)
- Image-Based Analysis of a Human Neurosphere Stem Cell Model for the Evaluation of Potential Neurotoxicants (Cytation 1, Cytation 5)
- Automation of HTRF® elF4E Kinase Assay Using a 3D Tumoroid- Based Cell Model (Cytation 1, Cytation 5, MultiFlo FX)
Visit our 3D Cell Culture application page to see more 3D Cell Culture applications from BioTek instruments.