Advances in Multimode Microplate Reading
May 2024
In scientific research, deeper insights often come from using microplate readers that offer multiple detection modes. The Agilent BioTek Synergy Neo2 hybrid multimode reader offers a powerful platform for myriad applications across various research fields. From the intricacies of cell migration to the technical nuances of proximity assays, the BioTek Synergy Neo2 empowers researchers with its distinctive capabilities.
Some conventional microplate reader designs struggle to integrate diverse detection modes efficiently. With independent light paths for each detection mode and a proprietary hybrid optical design for fluorescence detection, Synergy Neo2 overcomes this challenge. Through its advanced feature set and intuitive design, researchers can navigate intricate experimental setups with ease, to help reveal new dimensions of understanding.
In this edition of TekTalk, we provide an overview of the benefits of the hybrid technology available with the Synergy Neo2 for use in a diverse range of life science applications.
Hybrid optics
The hybrid design of the Synergy Neo2 includes a quad monochromator and a filter-based optical system. Using the optical system best suited for a specific workflow provides excellent performance efficiency, and versatility.
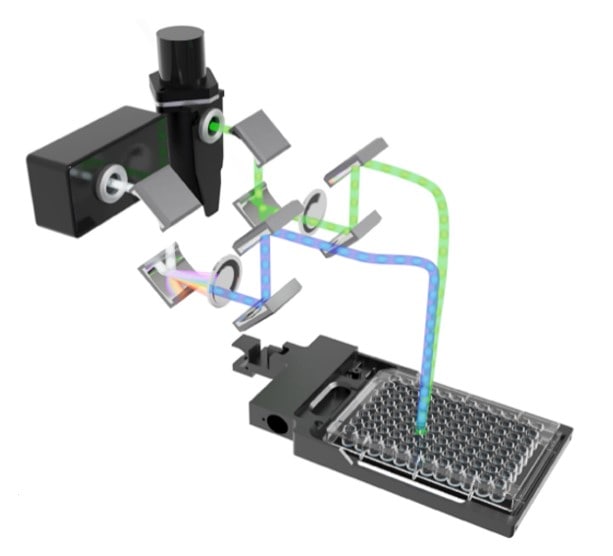
Quad monochromator optical system
Use the quad monochromator-based optics (Figure 1) in Synergy Neo2 for a very broad range of fluorescence intensity, glow and flash luminescence and UV-Vis absorbance assays. The quad monochromators enable absorbance measurements from 230 nm to 999 nm in 1 nm increments, accommodating nucleic acid protein quantification in microplates or in microvolume with the Take3 plate. Fluorescence excitation and emission wavelength selection is from 250 nm to 850 nm, with bandwidths user-selectable between 3 nm and 50 nm, in 1 nm increments. Higher bandwidths can increase sensitivity, while lower bandwidths can increase specificity. This capability is important for optimal detection conditions, especially with fluorophores that have a very narrow Stokes shift. The variable bandwidth enables fine-tuning of the channels to prevent crosstalk for multiplex assays like SNPs. Glow and flash luminescence measurements are also available using the monochromator optics for workflows in cell proliferation, gene expression, GPCR, ATP quantification, and more.
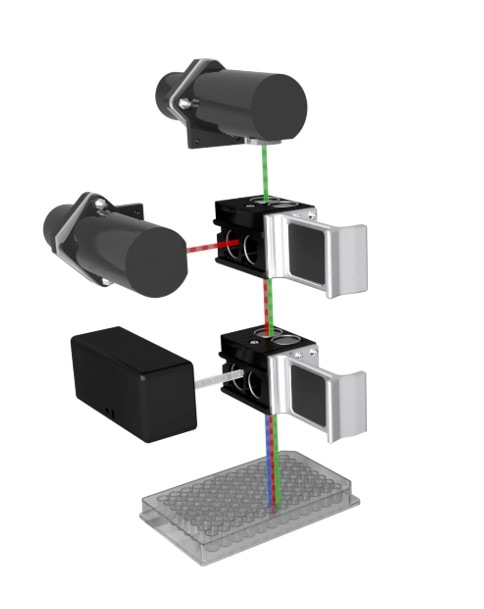
Filter-based optical system
Along with fluorescence intensity, filtered, glow and flash luminescence measurements, advanced detection modes such as time-resolved fluorescence (TRF), time-resolved fluorescence energy transfer (TR-FRET), fluorescence polarization (FP), BRET, and AlphaScreen are also available using the filter-based optics in Synergy Neo2 (Figure 2). The high-quality filter cubes and dichroic mirrors system enables high transmittance for best performance over a wide range of time-dependent and proximity assays. Top and bottom detection with high transmission and high sensitivity optimizes detection for cell-based assays using fluorescence or luminescence detection. Bottom filter detection can maximize sensitivity and minimize light loss for cell-based assays.
The filter cubes for Synergy Neo2 contain excitation and emission filters, along with dichroic mirrors, and/or polarizing filters in barcoded assemblies, to ensure correct filter cube selection and position. The extensive range of available filter cubes allows the best performance for many advanced fluorescence and luminescence workflows.
Featured Applications
Rapid Measurement of IgG Using Fluorescence Polarization

The accurate, rapid, and high-throughput measurement of IgG is essential in the development and subsequent manufacture of most therapeutic antibodies, as monoclonal antibodies are becoming increasingly dominant in biopharmaceuticals. This application note describes the use of the Agilent BioTek Synergy Neo2 hybrid multimode reader to rapidly determine IgG concentrations using the fluorescence polarization-based ValitaTiter assay provided by ValitaCell.
A Homogeneous FRET-Based HTS Assay for Quantification of pRb in Cancer Cell Lines

The field of cancer biology remains one of the most rapidly expanding areas of investigation in both industry and academia. Understanding disruptions that control pathways and checkpoints leading to uncontrolled tumor progression are key to understanding disease progression. Presented here is a novel HTRF cell-based assay that quantifies endogenous phosphorylated retinoblastoma protein as a readout of the G0 /G1 cell phase transition.
Tek Tips
1. Optimize proximity assays using FRET-based measurements
Proximity assays use energy transfer from a donor to an acceptor moiety. FRET based assays employ two fluorescent molecules with overlapping emission and excitation wavelengths in a manner such that the excitation of the donor will result in emission from the donor molecule and the acceptor. While the FRET signal obtained from the acceptor molecule’s emission is generally considered the more important result, information can also be gleaned from the donor molecule’s emission signal. While the dual-photomultiplier (dual-PMT) configuration allows for the simultaneous capture of both emission signals it is important that both are in the dynamic range of the PMTs. In general, the donor molecule emission will be significantly greater than that observed from the FRET reaction so the gain setting of the two PMTs needs to be set differently to reflect this. While the gain setting for each can be set manually, the AutoScale option in the Gen5 software will automatically integrate the plate to identify the highest well and set the gain appropriately. The Synergy Neo2 also offers an extended dynamic range that extended the signal range 100-fold.
2. Improve the accuracy of FP assays by setting the G factor
Fluorescence polarization (FP) is in essence an assay that measures changes in size. Fluorescence polarization is measured using the ratio of the fluorescence emission returned through two polarizing filters, one parallel (∥) to and one perpendicular (⊥) to the plane of polarized excitatory light. The formula used to calculate fluorescence polarization (P) uses an instrument and assay correction factor, referred to as G. To obtain useful and comparable data it is necessary that this correction factor be adjusted on an instrument-by-instrument basis. Small differences in the angle of the polarizing filters of different instruments can often lead to different results. Likewise, assay buffer that have viscous agents such as glycerol can affect results. It is best to adjust the G-factor against a known standard such as sodium fluorescein in the buffer system employed. This small molecule rotates freely in solution and has a reported mP value of 35. The G-factor can either be set prior to running an experiment by testing a standard independently or by dedicating specific wells on the test plate. The Synergy Neo2 in combination with Gen5 will use these wells to automatically set the PMT gain and G-factor to achieve a requested polarization value.
3. Utilize ratiometric FRET readouts for better data interpretation
The dual signal output of FRET based assays can also be used to correct for well-to-well pipetting variances, as well as increase the assay window. Rather than plotting the change in FRET signal against agonist compound concentration directly, plotting the ratio of the FRET signal to that of the donor molecule fluorescence against drug concentration can often have several advantages. The ratiometic nature of the determination often corrects for pipetting errors in assay reagents. With large FRET signals changes both the Acceptor emission and donor emission will change significantly in opposite directions with drug concentration. This bifold change will dramatically increase the assay window in terms of fold-change.
Product Spotlight
Synergy Neo2 Hybrid Multimode Reader
BioTek Synergy Neo2 is an ideal platform to measure a variety of proximity assays. In addition to the xenon flash lamp, the Synergy Neo2 can be configured with a 337 nm nitrogen laser for TR-FRET reactions such as the HTRF® or THUNDER™ assays. Likewise, AlphaScreen assays can be enabled with an optional 100 mW, 680 nm diode laser. The multiple light sources along with multiple PMTs allow for simultaneous capture two separate emissions. This fiber-less design provides direct illumination for very strong sample excitation which guarantees the highest levels of sensitivity. The optical system uses barcoded filter cubes containing dichroic mirrors and deep blocking bandpass filters for error free determination of excitation and emission. The reader is controlled, and the data reduction performed by Agilent BioTek Gen5 software. This combination of assay technology, software and instrumentation provides an ideal solution for high-throughput detection.
Featured Videos
Agilent BioTek Synergy Neo2 Hybrid Multimode Reader

Webinar OnDemand
Unlocking Discovery: The Agilent BioTek Synergy Neo2 Hybrid Multimode Reader for Next-Level Target-Based Research and Screening
Presenter: Charles William Amirmansour, Ph.D., Global Business Development Manager, Agilent
Watch this engaging webinar where we delve into the cutting-edge capabilities of the Synergy Neo2. Designed to address the diverse landscape of drug discovery and life sciences research, this innovative platform transcends traditional multimode microplate reading boundaries.
During this dynamic session, we’ll explore the proprietary hybrid design of the Synergy Neo2, showcasing its versatility across a multitude of applications. From fluorescence, luminescence, and absorbance assays to advanced applications including time-resolved fluorescence, fluorescence polarization and AlphaScreen, this multimode microplate reader empowers researchers with the tools to unlock new frontiers in scientific
Other Resources
- Monitoring of Protein Kinase Activity Using Next Generation CSox-based Substrate Sensors
- Investigation of Protein: Protein Interactions (PPI) using a Novel Bioluminescence Resonance Energy Transfer (BRET) Proximity based, High Throughput Screening Assay
- Kinetic Degradation Profiling of IMiD Molecular Glues and Their Target Families
- Fluorometric Quantitation of dsDNA using PicoGreen