Neurobiology Cell Analysis
November 2024
Neurobiology is a rapidly advancing field with significant implications for understanding brain function, neural development, and neurological disorders. Recent advancements in cell analysis techniques, such as high-resolution automated imaging and precise metabolic measurements, have revolutionized our ability to study neural cells in detail. These techniques are crucial for unraveling the complexities of neural health, synaptic interactions, and cellular metabolism, which are fundamental to both basic research and therapeutic development.
Automated imaging techniques offer significant advantages over conventional techniques. Widefield fluorescence and brightfield imaging methods provide an effective strategy for characterizing 2D neuronal cell models, while confocal imaging provides high-resolution, three-dimensional images of neural cells. Additionally, Seahorse XF metabolic assays enable real-time analysis of metabolic function in live cells. Integrating these techniques enhances throughput, data integration, and accuracy accelerating discoveries in neurobiology.
In a recent TekTalk edition, we featured the Agilent BioTek Neurite Outgrowth module, which offers a fully automated solution for quantifying neurite outgrowth using either fluorescence-based formats or label-free live cell approaches. This TekTalk explores additional neurobiology applications and solutions, including approaches for measuring treatment-induced changes in neuronal cell health and function using both 2D and 3D cell culture systems. We also present tips for achieving accurate results using cutting-edge automated cell analysis tools and optimized cell culturing techniques.
Featured Application Notes
Image-Based Analysis of a Human Neurosphere Stem Cell Model for the Evaluation of Potential Neurotoxicants
This application note details the use of a 3D neurosphere model composed of human neural stem cells for developmental neurotoxicity testing. It highlights the application of automated imaging and analysis using the Agilent BioTek Cytation 5 cell imaging multimode reader. This system enables detailed tracking of neurosphere growth, differentiation, and response to neurotoxicants through brightfield and fluorescence microscopy. The integrated Agilent BioTek Gen5 software facilitates precise, high throughput assessments by automatically analyzing changes in spheroid size and fluorescence signals, thus providing a robust and efficient method for evaluating potential neurotoxic effects.
Glycolytic Suppression - The Impact of Metabolic Modulation on Neural Stem Cell Fate
Assay designs for induced pluripotent stem cell (iPSC) differentiation towards neural progenitor cells (NPC) can be challenging. This application note investigates the metabolic drivers impacting neuronal stem cell differentiation, with respect to speed and efficiency. Modulating iPSC metabolism by replacing glucose with galactose promoted switching from a glycolytic to an oxidative phosphorylation phenotype that correlated with increased NPC differentiation efficiency.
Featured Posters
Metabolic Measurements of Disease-relevant Human iPSC-derived Cells Using Seahorse XF Assay Technology
Seahorse XF cell analysis provides sensitive label-free measurements that are useful in detecting changes in mitochondrial function between different human iPSC-derived neural cell types, as well as between wild-type and disease-mutation containing cells. The combination of these two technologies is a powerful tool for disease modeling.

Flexible Automated Platform for Multimodal Image-Based Neurite Outgrowth Analysis
Neurite outgrowth assays provide insights into the key phenotypic changes in neuronal models used across developmental biology, neurotoxicity, degenerative diseases, and neuroregeneration research areas. Automated approaches advance neurite outgrowth studies by improving scale, efficiency, and reproducibility.
Customer Story - Anatomi Corporation
Time to Accelerate the Pace of Neurobiology Research
At the University of Minnesota’s Stem Cell Business Incubator in Minneapolis, Anatomi Corporation has commercialized a technology, known as the Chrono™ platform. The technology eliminates bottlenecks so that researchers can focus on their research goals.
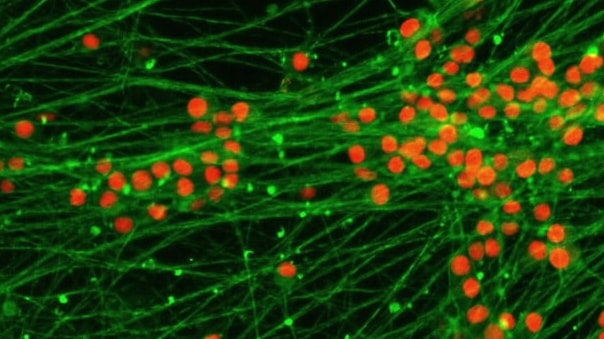
Tek Tips
Optimizing Consistency and Reproducibility in 3D Neurosphere Cultures for Neurotoxicity Testing
When working with human neurosphere stem cell models for neurotoxicity testing, it is essential to ensure the consistency and reproducibility of your 3D cultures. Start by using high-quality neural stem cells and maintain them under optimal conditions to prevent premature differentiation. Use specialized spheroid microplates to facilitate the formation and maintenance of uniform neurospheres. Regularly monitor the neurospheres for proliferation and differentiation markers using immunofluorescence techniques to confirm their multipotency and differentiation capabilities. Also, when exposing neurospheres to potential neurotoxicants, ensure precise dosing and consistent media changes to maintain the health of the cultures. If multiple neurospheres are present in each sample well, or if samples reside at random locations within each well, consider setting beacons marking the X, Y, and Z location of samples of interest for efficient image capture using the BioTek Gen5 image capture and analysis software tools. While 20x to 40x confocal images deliver deep insight into 3D cell culture models, 4x images are often sufficient for measuring the size of neurospheres and capturing considerable outgrowth/neuronal projections that develop over time within a single field of view.
Strategies and general guidance for improving the yield and quality of NPCs
Optimize cell density:
Start with an optimal cell density to ensure that cells have enough space to grow and differentiate without becoming overcrowded. Overcrowding can lead to nutrient depletion and increased metabolic stress, which can negatively impact differentiation efficiency.Use high-quality reagents:
Ensure that all reagents, including media, supplements, and growth factors, are of high quality and consistent batch-to-batch. This consistency is crucial for reproducible results.Monitor cell health:
Regularly check the health and morphology of the cells under a microscope. Healthy cells should have a uniform appearance and show expected morphological changes during differentiation.Control environmental conditions:
Maintain strict control over environmental conditions such as temperature, humidity, and CO2 levels. Fluctuations in these parameters can cause metabolic stress and affect differentiation outcomes.Implement proper controls:
Include appropriate positive and negative controls in your experiments to validate the differentiation process and ensure that observed effects are due to the experimental conditions and not external factors.Document protocols thoroughly:
Keep detailed records of all experimental procedures, including cell culture conditions, media changes, and any deviations from standard protocols. This documentation is essential for troubleshooting and replicating experiments.Regularly validate differentiation markers:
Use immunocytochemistry or flow cytometry to regularly validate the expression of key differentiation markers. This validation helps confirm that the cells are differentiating as expected and allows for early detection of any issues.Minimize mechanical stress:
Handle cells gently during media changes and passaging to minimize mechanical stress, which can negatively impact cell health and differentiation efficiency.
Product Spotlights
Agilent BioTek Cytation cell imaging multimode readers
The Agilent BioTek Cytation cell imaging multimode reader family includes inverted, upright, and spinning disk confocal imaging. Brightfield, color brightfield, phase contrast, transmitted light, and fluorescence channels are available depending on the product and configuration. Air and water immersion objectives, plus integrated environmental controls enable a variety of microscopy workflows, including live-cell kinetic studies. The Agilent BioTek Gen5 software for imaging and microscopy and its application-specific modules expand analysis capabilities for neurite outgrowth, object-tracking, spot-counting, and more. Multimode microplate reading modules for Cytation make it ready for any assay.

Agilent Seahorse XF Pro analyzer
Seahorse XF Pro analyzer measures and reports the oxygen consumption rate (OCR), proton efflux rate (PER) or extracellular acidification rate (ECAR), as well as ATP production rates of live cells in a 96-well format. This analyzer enables real-time measurement of glycolytic and mitochondrial function along the neural progenitor cell differentiation, providing better understanding of the interrelationship between metabolic poise and differentiation. It also represents a unique tool for characterizing the metabolic differences between iPSC-derived cell types and the corresponding disease models.
Webinars OnDemand
Advancing Neurobiology Research: Quantify Neurite Outgrowth with a Powerful Automated Solution
Neurite outgrowth assays provide a means to investigate key neuronal functions in vitro and advance both fundamental and clinical neuroscience research. In this interactive webinar, we present important considerations for conducting neurite analysis studies and introduce a powerful quantitative imaging solution for a broad range of neurite outgrowth applications.
Learning About the Mitochondrial Role in a Lysosomal Storage Neurodegenerative Disorder
CLN7 neuronal ceroid lipofuscinosis is an inherited lysosomal storage neurodegenerative disease highly prevalent in children. In her webinar, Dr. García Macía will discuss how failure in autophagy causes the accumulation of structurally and bioenergetically impaired neuronal mitochondria in the mouse model of CLN7 disease, and how the mitochondrial reactive oxygen species (mROS) in Cln7 disease neurons mediates glycolysis activation and contributes to CLN7 pathogenesis.
Additional Resources
- Neurite Outgrowth Module
- Evaluating Changes in Cell Metabolism in Neuroimmune and Neuropsychiatric Disorders
- Whole-Organism Imaging of Spinal Motor Neurons and Musculature with the Agilent BioTek Cytation C10 Confocal Imaging Reader
- Whole-Mount Immunofluorescence Imaging of Zebrafish Eye Structures
- Human SIRT5 variants with reduced stability and activity do not cause neuropathology in mice
- Metabolic Profiling of SH-SY5Y and Neuro2A Cells in Relation to Fetal Calf Serum (FCS) Concentration in Culture Media
For Research Use Only. Not for use in diagnostic procedures.
RA45589.5721759259