Cell Proliferation Assays
September 2024
Cell proliferation is the process by which cells multiply and increase in number over time. This biological phenomenon is governed by a delicate equilibrium between cell division, where one cell divides into two or more daughter cells, and cell death, which can occur through processes such as apoptosis or necrosis. The measurement of cell proliferation is a fundamental parameter in understanding cellular behavior and viability across various contexts.
Monitoring cell proliferation can provide essential insights into the normal developmental processes that are crucial for tissue formation and regeneration in multicellular organisms. It allows researchers to study how cells grow and divide to form organs during embryonic development or how damaged tissue is repaired.
Cell proliferation is also a critical aspect of disease research, cancer biology in particular. Malignant cells are characterized by uncontrolled growth and the ability to invade other tissues. Accurate determination of the rate at which cancer cells proliferate is pivotal in evaluating cellular characteristics under different regimes and the effectiveness and safety of cancer treatments.
In this TekTalk we’ve compiled an overview of the most common approaches for measuring mammalian cell proliferation, including advantages and drawbacks for each. Additionally, we describe effective strategies and powerful instruments for conducting cell proliferation studies, including a spotlight on live-cell imaging solutions (Figure 1) that present considerable advantages over conventional formats.
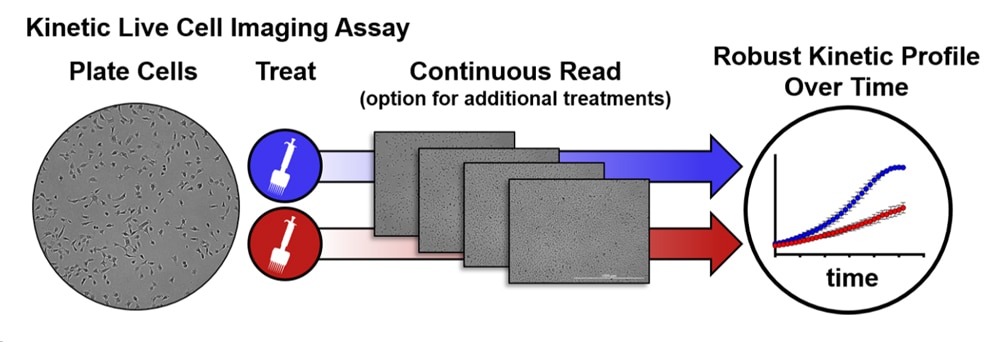
Featured Applications

Live Cell Proliferation and Viability
This application compendium covers brightfield and fluorescent microscopy methods to monitor cell proliferation and viability.
Automated Kinetic Imaging Assay of Cell Proliferation in 384-Well Format
This application note presents a fully automated image-based assay to monitor both the proliferation and cell death of a fibrosarcoma cancer cell line in response to multiple antineoplastic drugs in a high-throughput format.
Tek Tips
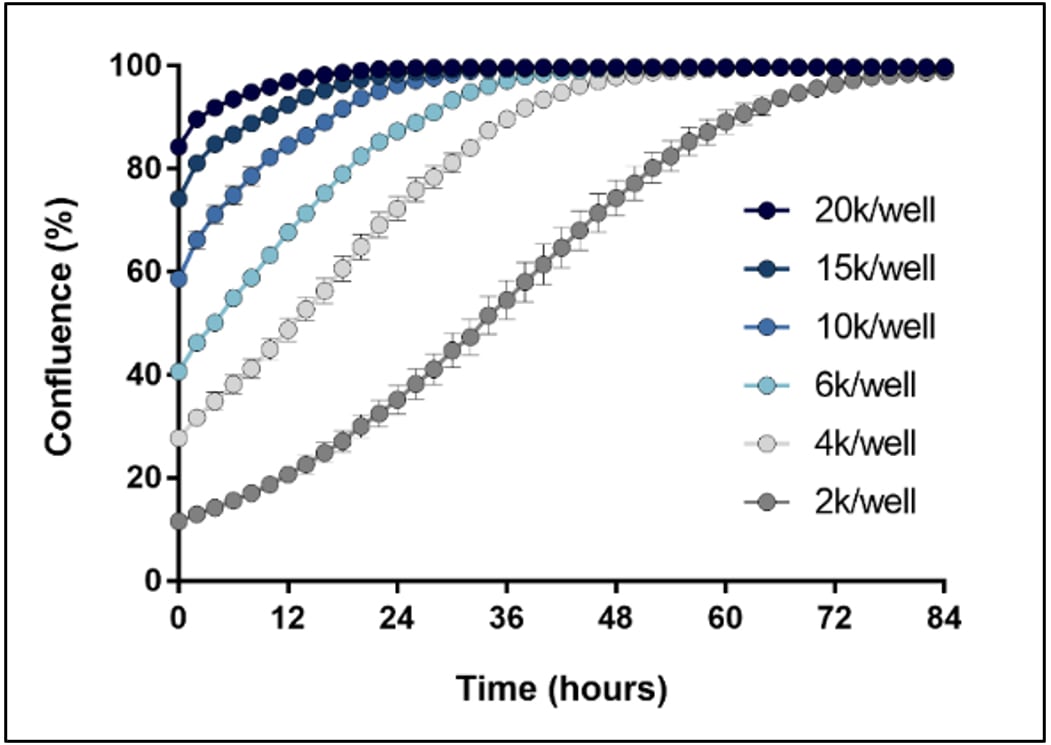
Optimize starting cell number for kinetic cell proliferation assays
An important component of proliferation assays is starting cell number, or cell density, within the microplate well. For instance, Figure 2 illustrates the relationship between starting cell density and the time required for the culture to reach 100% confluence. High starting cell densities result in sample values plateauing early within the time-course, prohibiting any further meaningful analysis, and limiting the ability to identify subtle differences across conditions revealed over time.
Method for ensuring even cell distribution for accurate evaluation of proliferation
Even distribution of cells within the sample vessel, especially microplates, is an important contributor to achieving accurate and reproducible cell proliferation assays. Placing the sample vessel directly into the culture incubator immediately after seeding will often lead to a higher density of cells at the perimeter of the culture chamber compared to the center, which can introduce variability and artifacts into the assay. This effect is due to convection currents created within the culture medium caused by the relatively rapid change in temperature. To reduce this effect, culture vessels should be kept at room temperature for approximately 30 minutes after cell seeding, allowing cells to evenly settle down to the culture surface before transferring the vessel to a cell culture incubator.
Review of Common Cell Proliferation Assays
1. Metabolic Activity Assays: Homogenous assays for measuring cellular energy levels within a cell sample (e.g., MTT and CellTiter-Glo). Typically conducted on a microplate reader.
- Convenience: Quick, easy, and straightforward detection.
- Throughput: Automation friendly, up to 1536-well microplate format.
Strengths:
- Endpoint Readouts: Typically provide only endpoint readouts.
- Indirect measurement: Can be influenced by factors other than proliferation, like cell health and metabolic activity.
- Cytotoxicity: Some compounds (e.g., MTT) can be cytotoxic over time.
Weaknesses:
Agilent application notes
2. Cell Proliferation Markers: This method detects specific proteins expressed during cell proliferation, such as Ki-67, PCNA, and phospho-histone H3. Typically conducted using fluorescence microscopy.
- High Content: Provides detailed information.
- Multiplexing: Can be combined with other assays for comprehensive analysis.
Strengths:
- Endpoint Readouts: Typically provide only endpoint readouts.
- Cost: Requires specific, often expensive antibodies.
- Processing steps: May need additional steps for detection.
Weaknesses:
Agilent application notes
3. DNA Synthesis Assays: This approach measures the incorporation of nucleotide analogs into newly synthesized DNA, using techniques like BrdU and EdU assays. Typically conducted using a microplate reader or flow cytometer.
- Versatility: Suitable for various applications (e.g., immunohistochemistry, flow cytometry).
- Non-Radioactive methods: Easy-to-handle alternatives are now available.
Strengths:
- Endpoint Readouts: Typically provide only endpoint readouts.
- Radioactive Methods: Require special handling and disposal.
Weaknesses:
Agilent application note
4. Dye Dilution Assays: This method uses fluorescent dyes (e.g., CFSE) to label cells. As cells divide, the dye dilutes, and the reduction in fluorescence intensity measures cell proliferation. The assay is typically evaluated using a flow cytometer.
- Single-Cell Resolution: Detailed insights into heterogeneous populations.
- Non-Radioactive: Avoids issues with radioactive methods.
Strengths:
- Cell Viability: Some dyes may affect cell viability/function.
- Technical Complexity: Requires careful optimization.
- Limited Long-Term Tracking: Fluorescent signal diminishes below detection levels over time.
Weaknesses:
Agilent solutions for dye dilution assays
5. Live-Cell Imaging: Advanced imaging techniques allow for real-time monitoring of cell proliferation. This method provides detailed insights into cell behavior and division over time.
- Direct readout: Measures changes in cell number over time
- Data normalization: Ability to account for variability in starting cell number across treatments and replicates.
- eal-Time Monitoring: Live cell imaging allows for continuous observation of cell proliferation over time, providing dynamic data on cell growth and behavior.
- Non-Invasive: Does not require the destruction of cells, allowing the same population to be monitored throughout the experiment.
- High Throughput: Automated systems can handle multiple samples simultaneously, making it suitable for large-scale studies.
- Quantitative and Qualitative Data: Advanced software can provide precise measurements of cell confluence, cell count, and other parameters, reducing subjective bias.
- Versatility: It can be used with various cell types and conditions, including label-free and non-cell-perturbing fluorescent label-based approaches.
Strengths:
- Cost: High-quality live cell imaging systems and associated software can be expensive compared to microplate readers.
- Data Management: The continuous acquisition of images generates large amounts of data, necessitating robust data storage and management solutions.
- Environmental Control: Maintaining optimal conditions (e.g., temperature, CO2 levels) during imaging can be challenging for conventional microscopes.
- Potential Artifacts: Prolonged exposure to light and/or fluorescent labels can introduce artifacts or affect cell viability.
Weaknesses:
Agilent Solutions for live cell imaging assays
Product Spotlights
BioTek BioSpa Live Cell Analysis System
The BioTek BioSpa live cell analysis system offers unique benefits for a wide variety of live cell imaging applications in up to 8 microplates or other labware. With 5 imaging modes, the system enables a wide range of microscopy applications.

Additional Resources
Resources for conducting 3D cell proliferation assays
Three-dimensional (3D) cell culture systems are an increasingly utilized model for studying human disease across a broad range of applications, including cell proliferation. Agilent BioTek automated imaging systems, including the Cytation C10 confocal imaging reader, provide flexible platforms that enable detailed quantitative analysis of 3D samples.
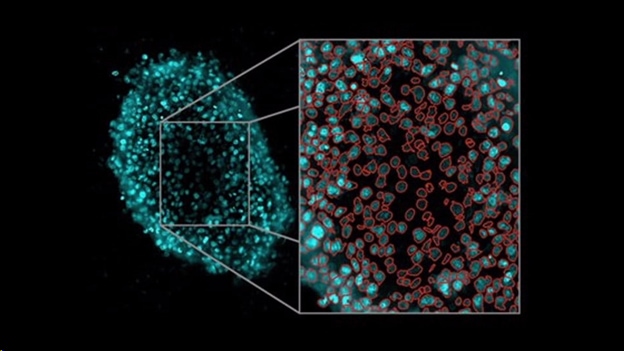
Application Note
Webinars
- A Practical Guide for 3D Cell Culture Systems and Optimizing Spheroid Imaging Assay - Part 1 of 2
- A Practical Guide for 3D Cell Culture Systems and Optimizing Spheroid Imaging Assay - Part 2 of 2
- High-Throughput Imaging of 3D Engineered Model Constructs
Resources for measuring bacterial and yeast cell proliferation
Agilent BioTek's unique portfolio of automated cell imagers and purpose-built microplate readers provide a full range of solutions for microbiology studies.