Access Agilent eNewsletter January 2016

Moving biologics to market with automated biopharma workflow solution
Vicki Pandey, Agilent Global Marketing Communications
Biologic recombinant proteins are highly complex molecules that require specific purification techniques, complex sample preparation, and often long analytical methods to achieve resolution and detection. High-throughput platforms and automated workflows are two ways to improve efficiency, increase throughput, and ensure precision. In this article, we review some examples of automated Agilent systems that can improve your workflows as you move biologics to market.
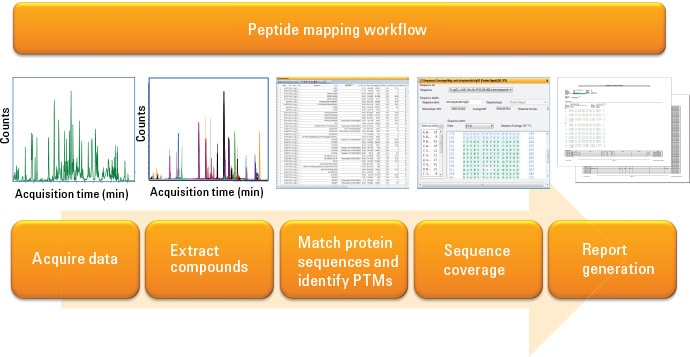
Figure 1. Agilent MassHunter BioConfirm offers automated peptide mapping data acquisition and analysis workflows.
Agilent MassHunter BioConfirm software delivers automated peptide mapping
A peptide mapping experiment may contain hundreds of peptides with various modifications. Automatic data processing improves experimental efficiency for data mining. Figure 1 shows the peptide mapping workflow provided by Agilent MassHunter BioConfirm software. After data acquisition, compounds are extracted automatically based on the chromatography characteristics. The table of compounds is then matched to the list of theoretical peptide digests with potential modification groups. The MS/MS spectrum of each peptide is interpreted by the b/y fragments of the peptides. A sequence coverage map is then compiled and the peptide mapping report automatically generated at the end of the workflow.
In this example, we used an Agilent AdvanceBio Peptide Mapping column in an Agilent 1290 Infinity LC and the Agilent 6550 iFunnel Q-TOF LC/MS with Agilent Jet Stream technology. To learn more about this automated peptide mapping application, download Agilent publication 5991-3600EN.
Agilent AssayMAP Bravo allows parallel processing of samples with low variability
An ever-increasing number of potential biotherapeutic proteins must be rapidly and accurately characterized so that data-driven decisions can be made to move the best candidates efficiently through drug discovery and development processes. This demands reductions in sample bottlenecks that in turn require more rapid, scalable, and reproducible methods to supplant current low-throughput and laborious manual methods with common workflows. These workflows include protein purification and quantification, whole protein mass determination, sequence coverage analysis, and characterization of posttranslational modifications.
The Agilent AssayMAP Bravo platform addresses these challenges by enabling the parallel processing of samples through a variety of LC/MS sample prep workflows, with low variability and minimal hands-on time.
Figure 2 illustrates a sample prep workflow on AssayMAP Bravo. A full plate of 96 samples, each containing 15 µg of mAb, was processed using affinity purification, in-solution digestion, and peptide cleanup. LC/MS produced extracted ion chromatograms for six proteotypic peptides, from which relative protein abundance could be inferred. CVs of 5 to 8% were calculated from EIC peak areas for all 96 samples, which indicated that reproducibility of the entire workflow was very high.
For this trial, replicates of mAb in particulate-free CCS were purified in parallel. The resulting peptides were purified and concentrated on C18 reversed-phase cartridges, resulting in samples ready for LC/MS analysis. We employed an Agilent 1260 Infinity Bio-inert Quaternary LC and 1290 Infinity LC equipped with Agilent Poroshell 300SB-C18 and AdvanceBio Peptide Mapping columns. A 6550 iFunnel Q-TOF LC/MS with Agilent Jet Stream technology delivered the required sensitivity. Explore further in Agilent publication 5991-4872EN and see how you can avoid process bottlenecks.
Agilent columns offer high-resolution analysis of complex glycan
Detailed characterization of the glycan profile of biopharmaceuticals, such as recombinant EPO (rhEPO), is essential as differences in glycosylation can affect their pharmacodynamics and pharmacokinetic behavior. The method of choice is typically hydrophilic interaction chromatography (HILIC) after labelling with 2-aminobenzamide (2AB) for sensitive fluorescence detection.
Whereas HILIC efficiently separates glycans according to hydrodynamic radius, it is insufficient to fully resolve the complex mixture of branched glycan structures that are present in samples such as EPO or fetuin. Fortunately, weak/strong anion-exchange chromatography (WAX/SAX) provides a highly orthogonal separation that depends on the number and arrangement of acidic monosaccharides in the glycan. To improve resolution and enhance peak capacity, a combination of WAX and HILIC separation can be used. First, a comprehensive WAX/HILIC 2D-LC setup was tested using fetuin. A 110 minute WAX gradient with an Agilent Bio WAX column was used for the first dimension, followed by a 30 second, second-dimension comprehensive HILIC run using a 4.6 × 50 mm HILIC column.
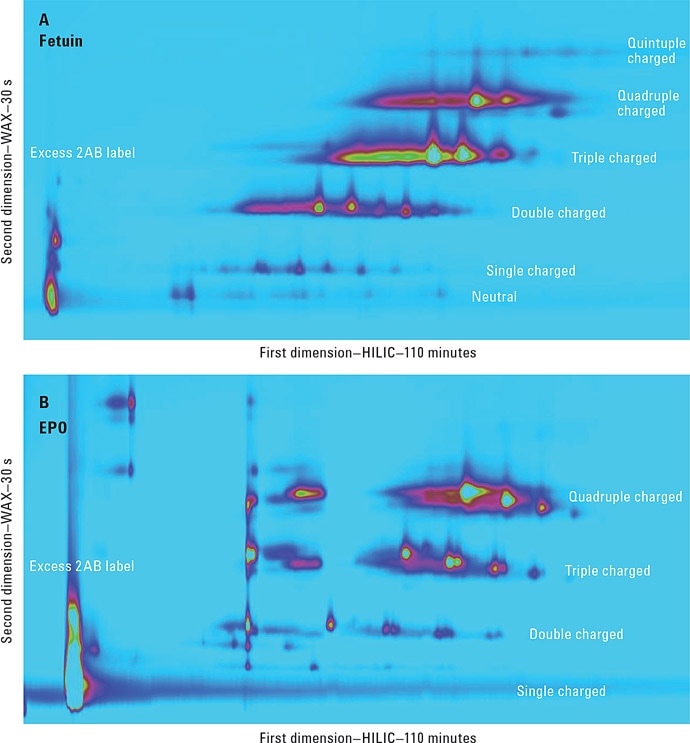
Figure 3. Comprehensive HILIC/WAX 2D-LC separation of fetuin and EPO, showing highly orthogonal separation. The ion exchange chromatography in the second dimension reveals the charge pattern of the glycans.
The comprehensive HILIC/WAX separation was used in an online 2D-LC setup, maintaining the highly orthogonal separation. Figure 3 shows the 2D-LC image from a HILIC/WAX 2D run. The 2D separation provides high peak capacity and resolution, and many of the coeluting peaks from the HILIC dimension are well separated by the WAX column.
The Agilent 1290 Infinity II 2D-LC solution, together with the 1260 Infinity Fluorescence Detector and the combination of HILIC and WAX columns, delivers excellent resolving power for highly complex glycans from therapeutic glycoproteins. You will find more details in Agilent publication 5991-5349EN.
Agilent solutions accelerate biopharma discovery and development
Whether you are looking to increase sample throughput, accelerate method development, automate sample preparation, or solve challenging biopharma applications, the highly modular Agilent approach gives you the flexibility you need to succeed. Explore a wide range of LC tools and solutions, as well as LC/MS instruments, for the automation of virtually any pharma-related liquid chromatography application. To discover more, contact your Agilent Representative who can help you identify the right tools for your application.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
Article Directory – January 2016
All articles in this issue
 What is in your beer? Find out using a Agilent J&W DB-624 Ultra Inert (UI) column with GC/MS static headspace solution
What is in your beer? Find out using a Agilent J&W DB-624 Ultra Inert (UI) column with GC/MS static headspace solution Moving biologics to market with automated biopharma workflow solution
Moving biologics to market with automated biopharma workflow solution Nobel Prize-winning lab uses Agilent equipment to accelerate natural product discoveries
Nobel Prize-winning lab uses Agilent equipment to accelerate natural product discoveries Tip: How to detect leachables from drug container closures in pharmaceutical products
Tip: How to detect leachables from drug container closures in pharmaceutical products Fast, accurate absolute-quantification of proteins and antibodies with Agilent 8800 ICP-QQQ
Fast, accurate absolute-quantification of proteins and antibodies with Agilent 8800 ICP-QQQ Chevron and Agilent collaborate on new multi-element crude oil analysis technology that delivers advantages in speed, cost, and safety
Chevron and Agilent collaborate on new multi-element crude oil analysis technology that delivers advantages in speed, cost, and safety Ask the Expert: Can I improve the chromatography of basic compounds on my older HPLC systems?
Ask the Expert: Can I improve the chromatography of basic compounds on my older HPLC systems? More pesticides identified using Agilent Pesticide DRS Screening GC/MSD Analyzer with new high efficiency source
More pesticides identified using Agilent Pesticide DRS Screening GC/MSD Analyzer with new high efficiency source Improved peak shapes for active semivolatiles with inert Agilent J&W DB-UI 8270D columns
Improved peak shapes for active semivolatiles with inert Agilent J&W DB-UI 8270D columns
Figure 1

Agilent MassHunter BioConfirm offers automated peptide mapping data acquisition and analysis workflows.
Figure 2
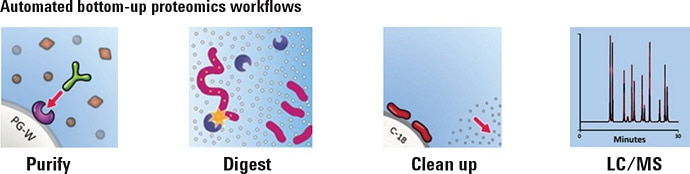
Workflow for antibody sample preparation.
Figure 3

Comprehensive HILIC/WAX 2D-LC separation of fetuin and EPO, showing highly orthogonal separation. The ion exchange chromatography in the second dimension reveals the charge pattern of the glycans.