TRS100 Quantitative Pharmaceutical Analysis System
The TRS100 provides fast, whole tablet or capsule content uniformity and polymorph screening for pharmaceutical finished-product testing and formulation development. A TRS100 system can reduce tests to seconds per sample, saving significant cost and speeding up your quality control testing workflow. Whole intact sample analysis means TRS100 avoids multistep consuming sample preparation stages required for conventional analysis. TRS100 workflows are sustainable as they use less analytical resources, solvents, and consumables.
The Agilent TRS100 offers:
- Bulk quantitative analysis of whole intact samples avoiding sample preparation
- No consumption of water or consumables during use phase
- Long lasting instrument with minimal maintenance
- Manufactured using renewable energy
- Instrument lifespan greater than 10 years
- Innovative analysis workflows with sustainable credentials

Additional Information
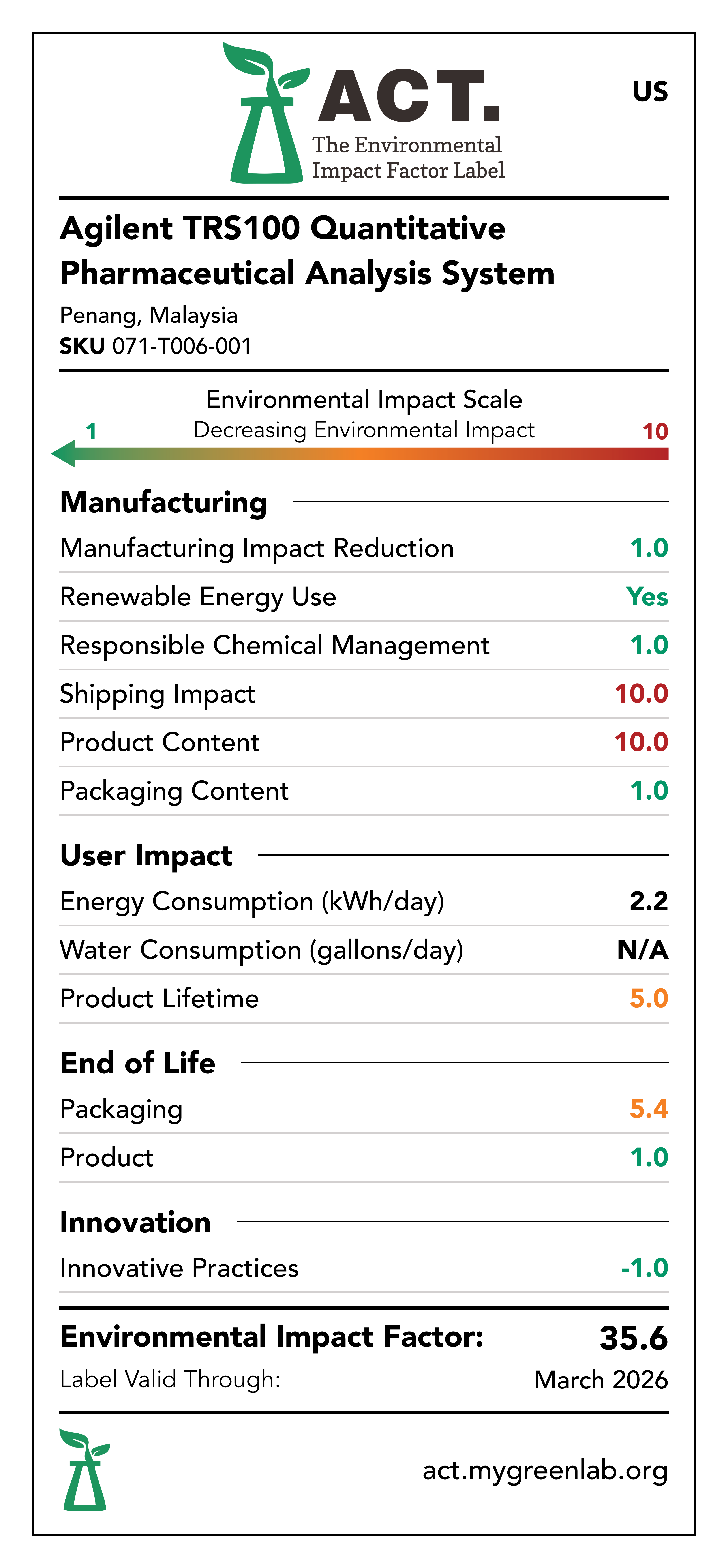
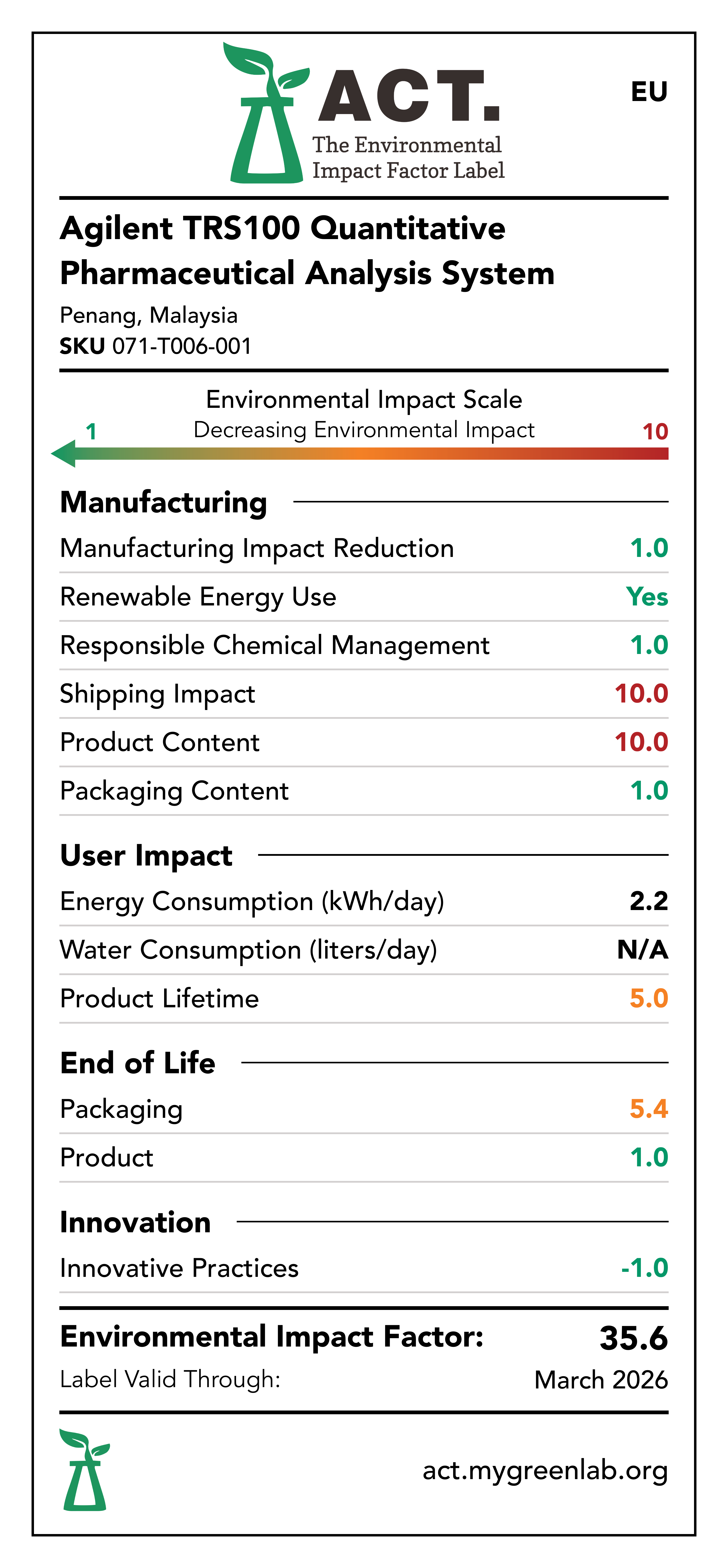
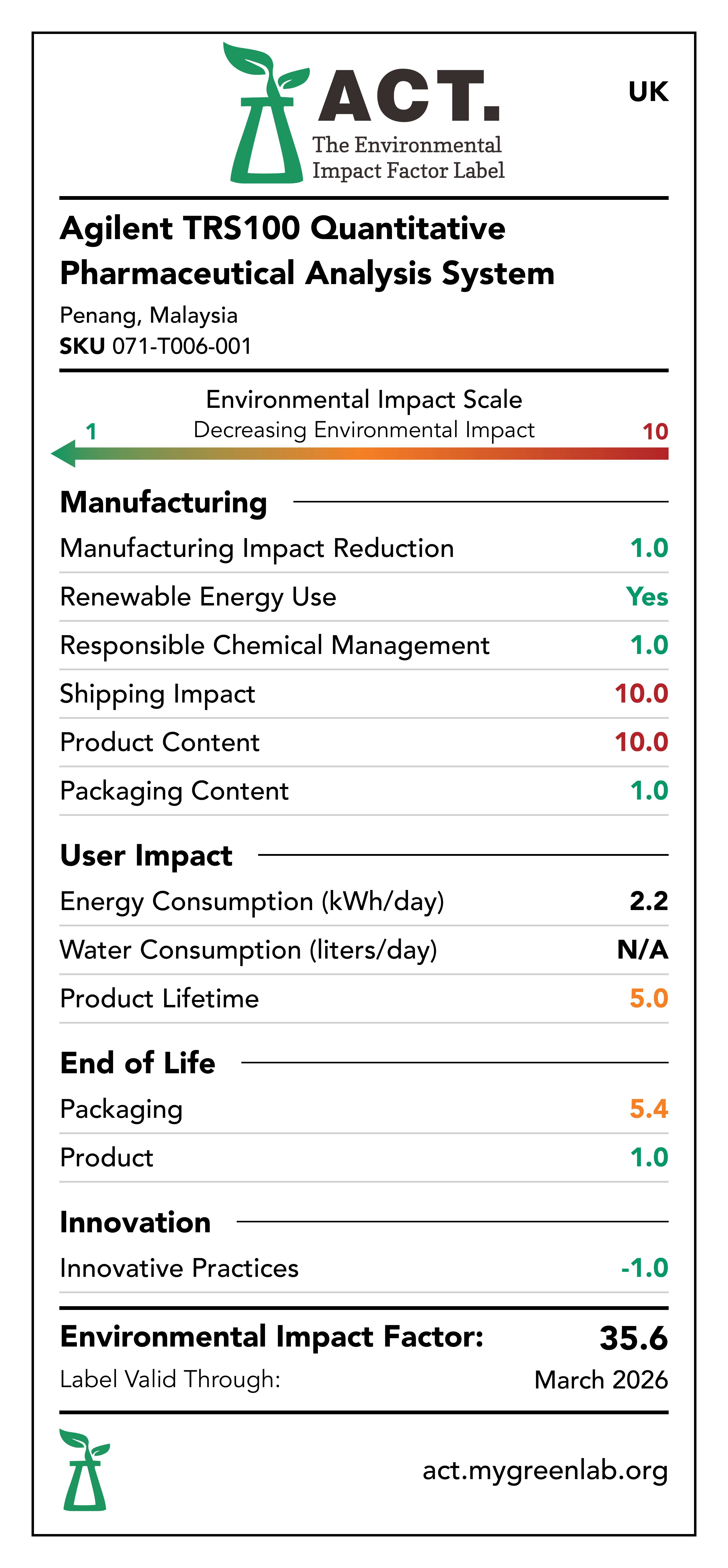
Manufacturing Impact Reduction
The Penang, Malaysia facility that manufactures the Agilent TRS100 Quantitative Pharmaceutical Analysis System has implemented measures to reduce energy consumption, water consumption, and waste generation within the last 5 years. These initiatives include movement sensitive lights, movement sensitive water faucets, and removal of single-use plastics.
Renewable Energy Use
The Penang, Malaysia facility that manufactures the Agilent TRS100 Quantitative Pharmaceutical Analysis System has a rooftop solar array.
Responsible Chemical Management
The Penang, Malaysia facility that manufactures the Agilent TRS100 Quantitative Pharmaceutical Analysis System has implemented a rigorous EMS policy and hazard communication plan. Additionally, the Agilent TRS100 Quantitative Pharmaceutical Analysis System is EU RoHS compliant and does not utilize any chemicals of concern.
Shipping Impact
The facility that manufactures the TRS100 Quantitative Pharmaceutical Analysis System is located in Penang, Malaysia with distribution centers in Memphis, Tennessee for the US Market and Ketsch, Germany for the EU and UK markets.
Product Content
The TRS100 Quantitative Pharmaceutical Analysis System consists largely of electronics, optical elements, plastics, and aluminum.
Packaging Content
The TRS100 Quantitative Pharmaceutical Analysis System is packaged using a wooden box.
Energy Consumption
The TRS100 Quantitative Pharmaceutical Analysis System consumes energy during use. It is assumed that the TRS100 Quantitative Pharmaceutical Analysis System would consume 2.2 kWh per day.
Water Consumption
The Agilent TRS100 Quantitative Pharmaceutical Analysis System does not consume water during use phase.
Lifetime Rating
Based on the available information regarding the lifespan of the Agilent TRS100 Quantitative Pharmaceutical Analysis System components, the device is estimated to have a lifespan greater than 10 years.
Packaging End-of-Life
The Agilent TRS100 Quantitative Pharmaceutical Analysis System is packaged using a wooden box. Wood is readily recyclable across all 3 markets: US, EU, and UK. Additionally, Agilent provides its customers with educational material that explains how to follow packaging disposal labels.
Product End-of-Life
The Agilent TRS100 Quantitative Pharmaceutical Analysis System can be returned at its end-of-life via Agilent's take-back program in all markets: US, EU, and UK.
Innovative Practices
The Agilent TRS100 Quantitative Pharmaceutical Analysis System is capable of quantifying the amount of active drug ingredient in whole intact pharmaceutical oral solid does forms, such as tablets and capsules. This workflow does not require sample preparation, avoiding multi-step and time consuming sample preparation steps which use solvents and consumables.