What are the advantages of LC/MS?
With the power of liquid chromatography separation and mass spectrometry detection, you can analyze the most complex samples.
LC/MS is:
- Reliable. Robust instrumentation delivers consistent results with complex and dirty samples.
- Multiplexed. You can add more dimensions to your data with multiple detector options (ultraviolet, evaporative light scattering, MS, etc.). What’s more, you can ionize with diverse ion sources (atmospheric pressure chemical ionization, electrospray, multimode, and more).
- Sensitive. Mass spectrometry can achieve unrivaled sensitivity with selected ion monitoring (SIM).
- Selective. Every molecule has unique fragmentation that can be matched to fragmentation libraries by collision induced dissociation (CID) and multiple reaction monitoring (MRM).
- Suitable for large-molecule analysis. Multiply charged ions enable the detection and characterization of very large molecules (>150 kDa).
- Quantitative. Agilent offers products well suited for the most demanding quantitative analyses, from routine testing to targeted research applications.
What is LC/MS used for?
LC/MS applications range from analyzing small molecules (like metabolites and carbohydrates) to the study of macromolecules (like proteins, peptides, and RNA/mRNA). Electrospray ionization used in LC/MS also enables large molecules to become multiply charged, effectively bringing their m/z ratio into the range of the instrument. For this reason, LC/MS is suitable for analyzing large molecules that can’t be measured by GC/MS.
Check out the LC/MS applications tab for some examples.
How can I decide what kind of instrument is best for my analysis?
To determine the best instrument, consider the molecular weight and polarity of your analytes, and whether your application will primarily be targeted analysis or unknowns determination.
What are some advantages of LC/MS compared to other detection methods (such as UV)?
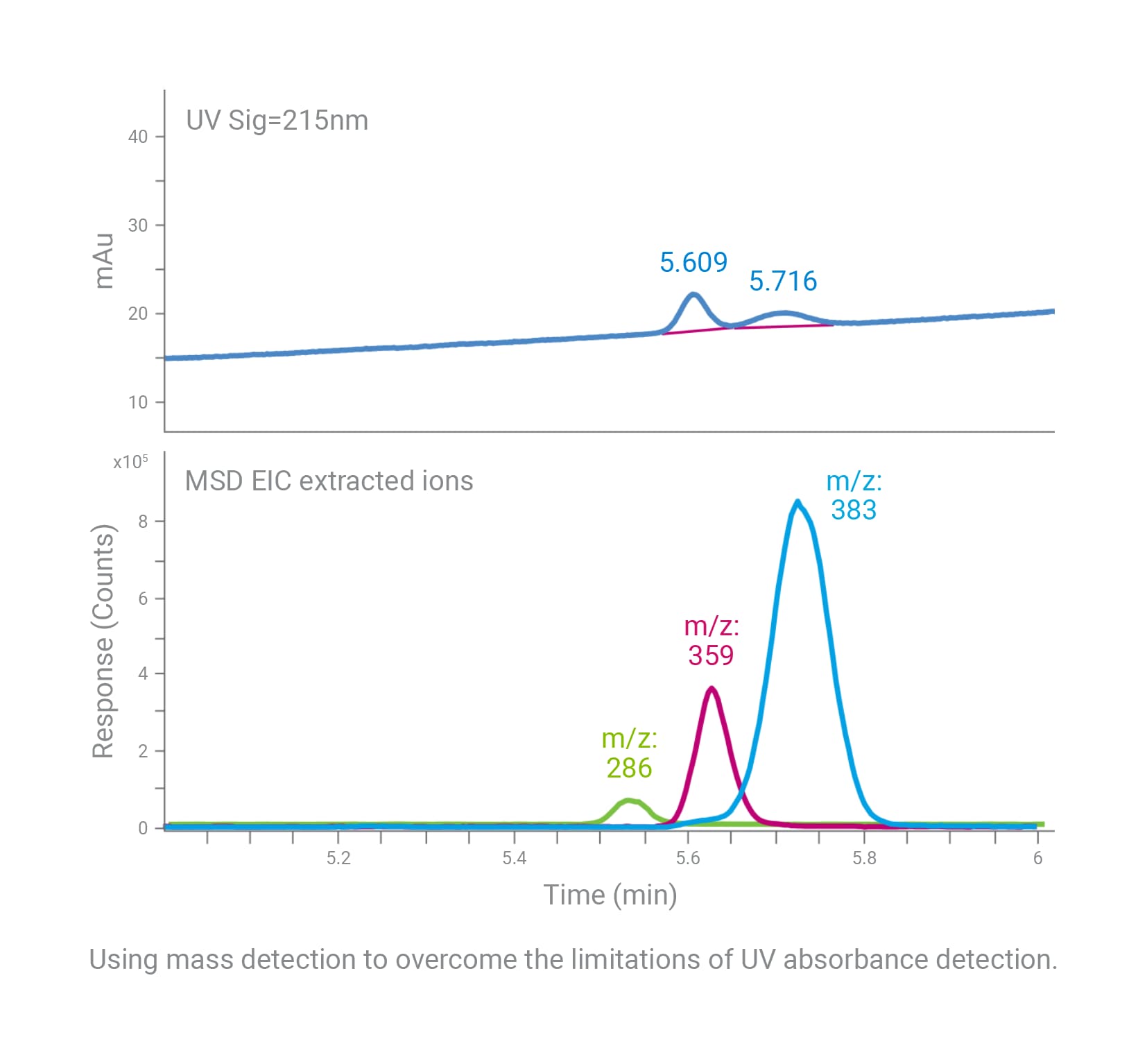
LC/MS systems can detect and measure compounds that are unresolved and unobserved with ultraviolet (UV) analysis. For impurity analysis, where compounds may coelute or have low UV absorbance, data from a single quadrupole mass spectrometer can boost confidence in your analysis. In addition, mass spectrometry enables users to identify and verify specific compounds and modifications of interest.
Using mass detection overcomes the limitations of UV absorbance detection. In this example, the mass spectrometer reveals an additional compound not resolved via UV analysis.
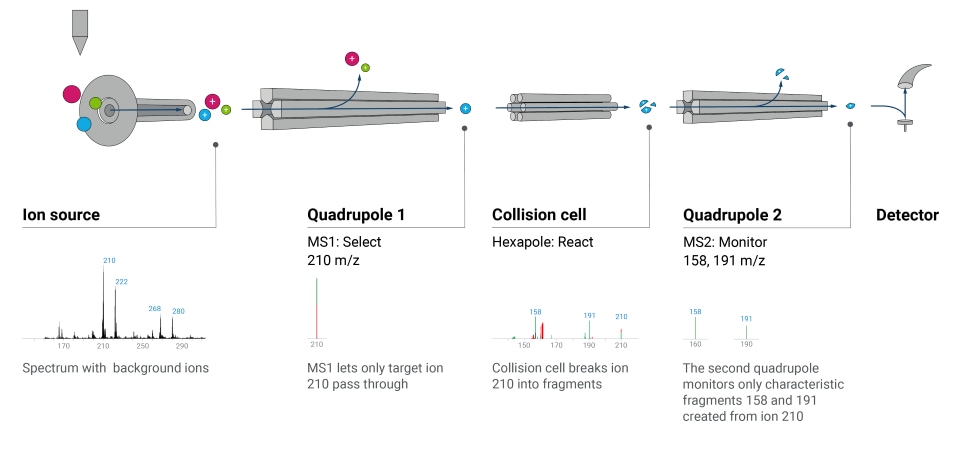
What’s the difference between single quadrupole and triple quadrupole LC/MS?
Single quadrupole LC/MS systems contain one quadrupole that can be used to analyze intact molecular ions and fragments created in the ion source. Triple quadrupole LC/MS/MS systems include an additional collision cell and quadrupole analyzer to enable LC/MS/MS analysis (as shown in image below).
LC/MS/MS may also refer to highly selective operational modes where more than one fragmentation and filtering step is performed, as with multiple reaction monitoring (MRM). During MRM, a mass filter is applied to the first quadrupole, further fragmentation occurs in the collision cell, and another mass filter is applied to the second quadrupole.
In addition, LC/MS/MS can refer to collision induced dissociation (CID) on an LC/Q-TOF system where the time-of-flight detector allows the second mass spectrometry (MS) stage.
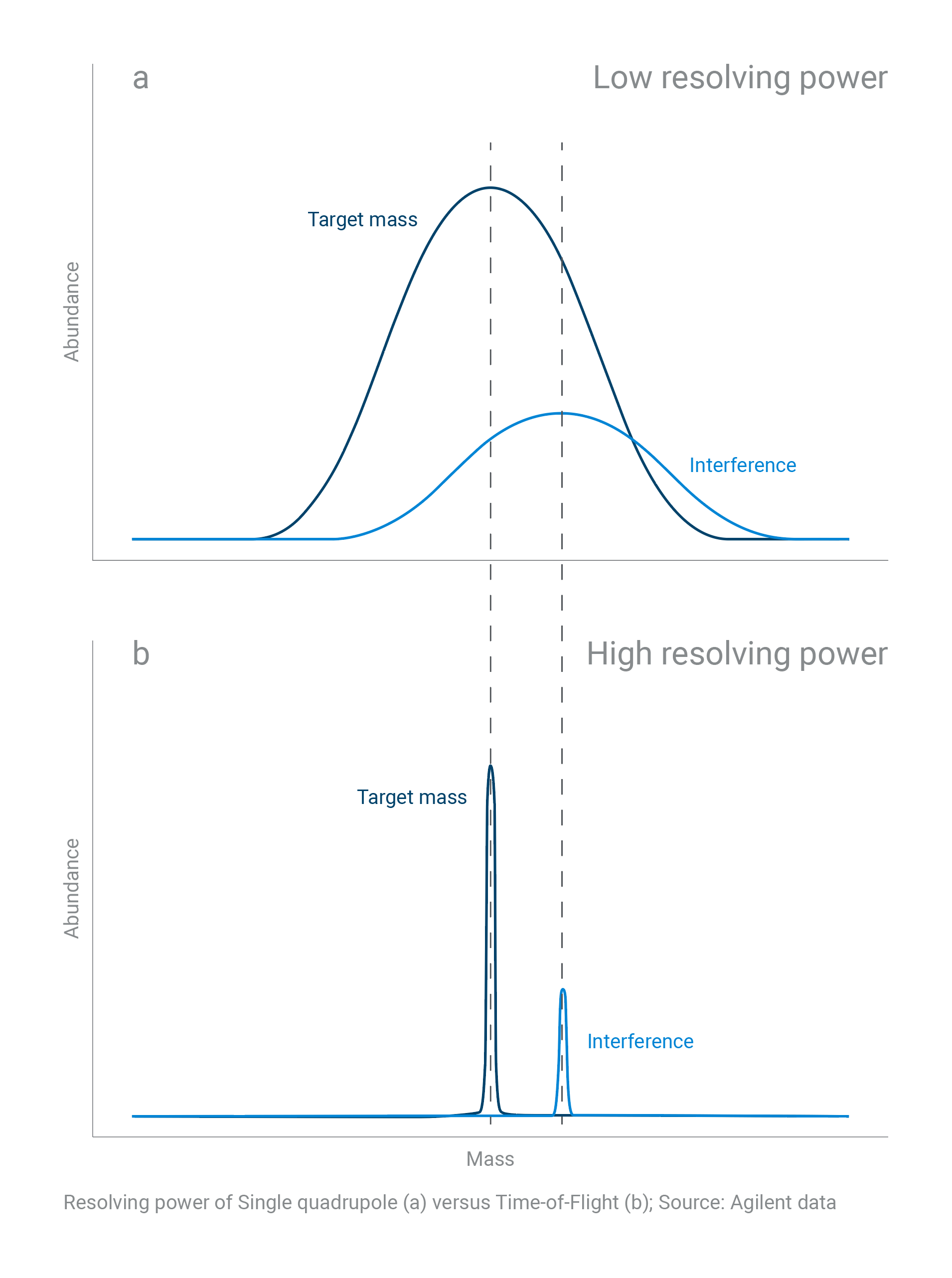
What’s the difference between a quadrupole and a TOF?
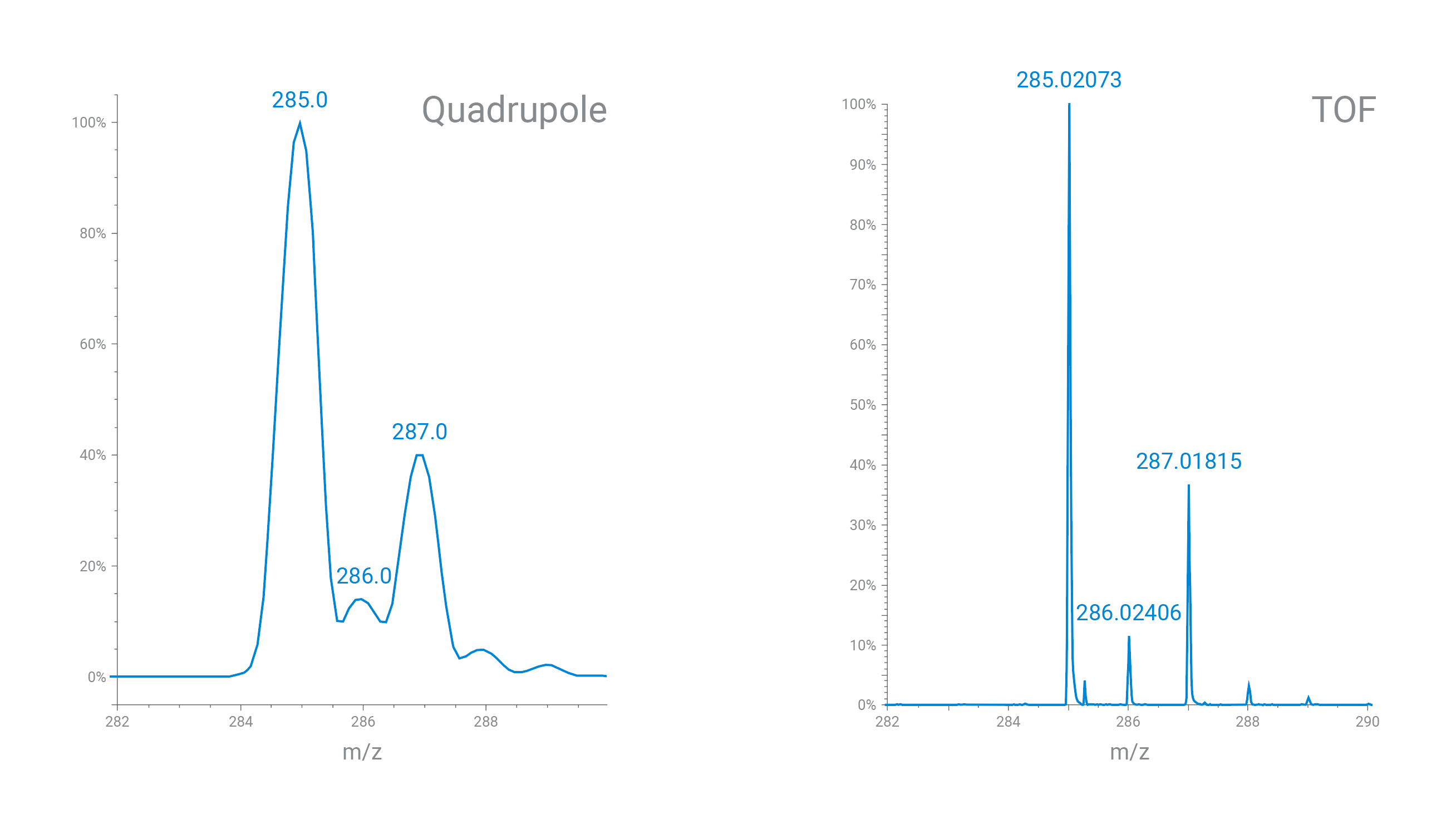
A single (or triple) quadrupole mass spectrometer delivers nominal mass (low resolving power). Time-of-flight (TOF) instruments can deliver accurate mass information (high resolving power).
A TOF mass spectrometer can positively confirm elemental composition through sufficient mass resolution and mass accuracy. With more resolving power (lower mass error), the number of possible molecular formulas shrinks, delivering a clearer, more accurate result.
The resolving power of a single quadrupole versus TOF mass spectrometer is shown in this analysis of sulfachloropyridazine.
What’s the difference between TOF and Q-TOF?
Adding a quadrupole and collision cell to a time-of-flight (TOF) mass analyzer forms a quadrupole time-of-flight (Q-TOF) mass spectrometer. A Q-TOF system performs MS/MS using the quadrupole, the hexapole collision cell, and the time-of-flight analyzer to produce spectra.
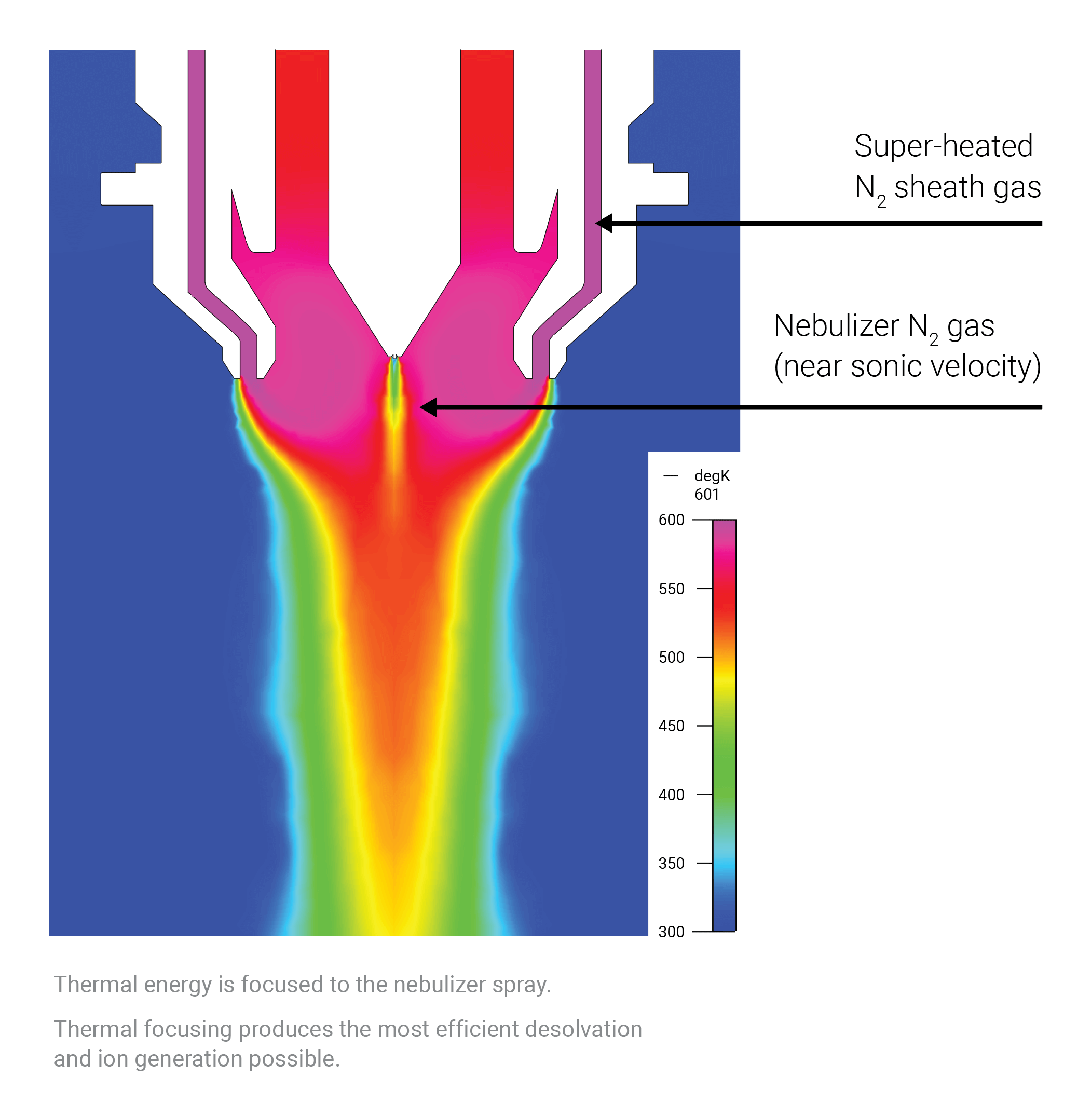
What makes Agilent Jet Stream technology unique?
The thermal gradient focusing technology used in the Agilent Jet Stream ion source extends electrospray ionization sensitivity for an array of applications and flow rates. This technology for high-sensitivity MS uses superheated nitrogen to improve droplet desolvation and ion generation for a stronger signal and reduced noise. It offers the highest sensitivity for most analytes, and a response that’s five-fold higher or more relative to standard electrospray ionization.
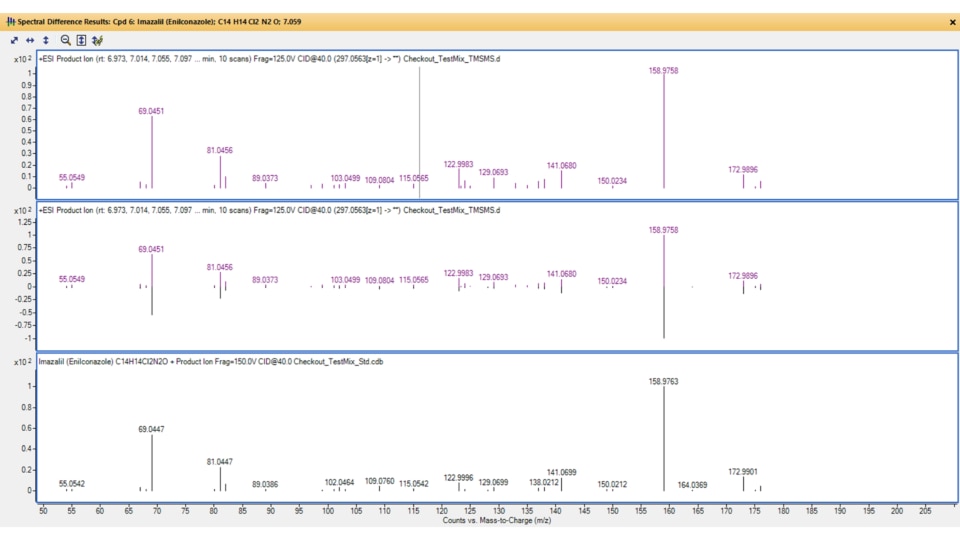
What are mass spectral libraries? What is their role?
Spectral libraries are mass spectra repositories that allow users to compare (or match) mass spectra acquired on high resolution LC/MS systems with a reference standard spectrum. Spectral libraries include associated compound and chemical structure information, such as the formula, mass, and identifiers (like the CAS and InChIKey).
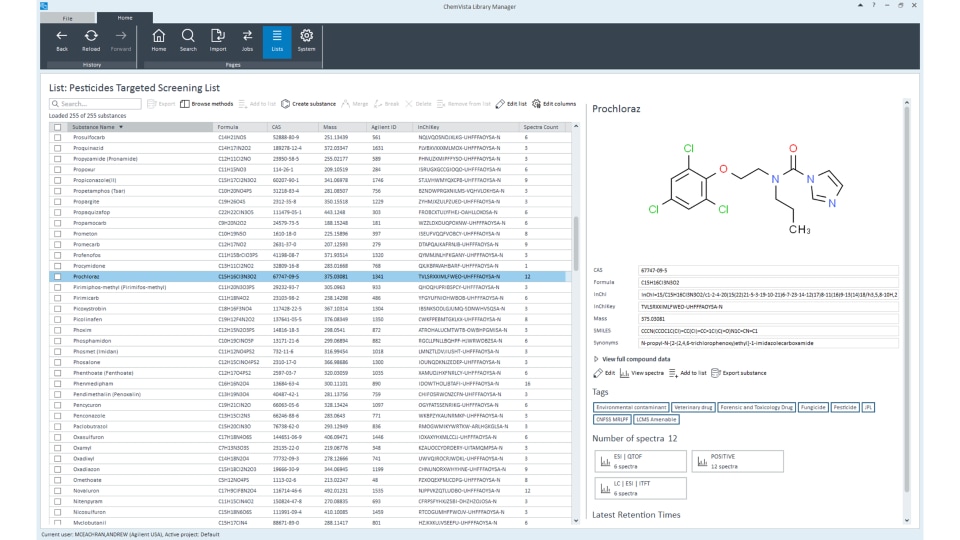
In practice, a library match score is calculated between the acquired spectrum and library spectrum, giving an indication of how well the two match (the higher the score, the more confident the match). Library matching aids in unknown identification and lends confidence to the compound identification process. You can screen for several thousand targets, such as pesticides, with a single run on a TOF or Q-TOF LC/MS instrument and accompanying spectral library.
Just as a museum curator extensively evaluates the integrity of each exhibit, Agilent deploys a stringent curation process to ensure the quality of its compound databases and spectral libraries.
Agilent ChemVista library manager software enables comprehensive spectral library management. It can also integrate compound, retention time, and mass spectra from multiple sources into one location, streamlining unknowns identification workflows in MassHunter data analysis software.
ChemVista includes extensive, curated LC/Q-TOF libraries and databases for food testing, environmental, extractables and leachables (E&Ls), and forensic toxicology applications.
How do I determine sensitivity or limit of detection in LC/MS?
Traditionally, a mass spectrometry system’s analytical sensitivity has been defined using the signal-to-noise ratio (S/N) as a metric. However, this approach is misleading since S/N values can be inflated based on how they are calculated. The instrument detection limit (IDL) is a more robust, reliable method for assessing detection limits and precision, giving you more confidence that your signal isn’t noise.
Learn more about IDL, how it’s calculated, and how you can use it to assess the performance of your LC/MSD, GC/MSD, triple quadrupole LC/MS, or GC/MS system.
Do LC/MS responses linearly correlate with concentration?
Most molecules measured by single or triple quadrupole LC/MS have linear regions in their response curve. As you get closer to the detection limit, the response becomes less linear, and drops further as you approach detector saturation. Generally, there can be three to four orders of linear range between extremes, but higher results are possible. In general, triple quadrupole instruments offer broader linear dynamic range than TOF/Q-TOF instruments.
What is high-throughput mass spectrometry?
High-throughput mass spectrometry (HTMS) is useful when hundreds or thousands of samples need to be analyzed in a short period. The limiting factor for throughput is often the chromatographic separation performed before MS analysis. To speed up LC separation, analysis times can be shortened through appropriate method choices (gradient, flow rate, column packing, and so forth). However, traditional LC/MS analyzes samples serially, which can leave a mass spectrometer idle and underutilized.
There are two easy ways to use your MS more efficiently:
-
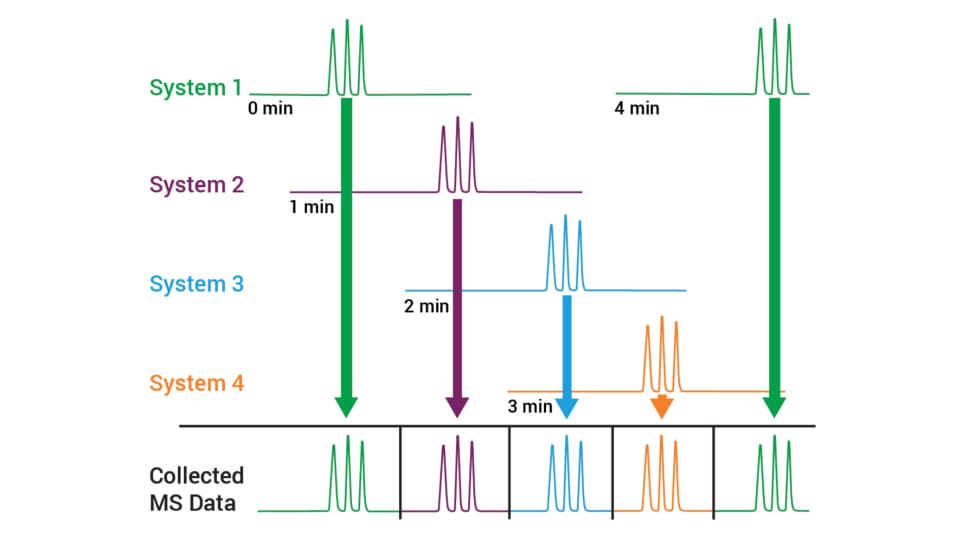
StreamSelect uses the same technology as traditional LC/MS, but replicates two, three, or four independent LC systems ahead of a single mass spectrometer. By performing staggered, offset injections, you can run up to four LC separations in parallel.
In a four-minute method, for example, we might only be interested in the one-minute window around the elution of peaks of interest. A significant portion of the chromatographic run contains no peaks of interest. Traditional LC/MS can complete one analysis in four minutes, while a four-stream StreamSelect system can complete four analyses in the same four minutes. This is equivalent to analyzing one sample per minute, a four-fold improvement in throughput.
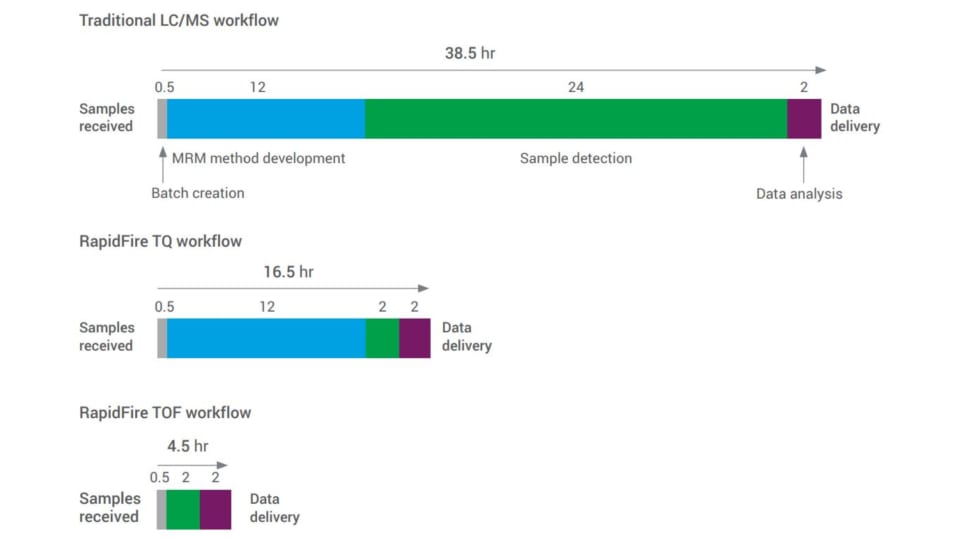
- Implementing RapidFire high-throughput LC/MS ahead of a mass spectrometer automates solid-phase extraction (SPE) and sample delivery from microplates to the mass spectrometers. By replacing the traditional LC separation step with SPE, the processing time for each sample is dramatically reduced from minutes to seconds. RapidFire typically operates at a rate of 8 to 15 seconds per sample, depending on the specific parameters of the solid phase extraction. For sample matrices that don’t require cleanup, bypassing SPE allows RapidFire to reduce data acquisition time to as little as two seconds per sample. This speed is 10 to 100 times faster than traditional LC/MS.