Access Agilent eNewsletter January 2017

ICP-MS and ICP-OES methodology enables comprehensive analysis and quantification of elemental extractables and leachables
Paige Solomon
Agilent Marketing Specialist
Jenny Nelson
Agilent Application Scientist
Andreas Tei
Agilent Global Pharma Marketing Manager
The analysis of extractables and leachables (E&Ls) as impurities in a wide range of drug products is critical to the pharmaceutical packaging industry. Several product recalls have been linked to potentially toxic materials originating from drug containers or parts of the packaging. E&Ls comprise a large variety of compounds that include:
- Plasticizers as potential endocrine disruptors
- Genotoxic compounds such as nitrosamines and polyaromatic hydrocarbons
- Toxic elements
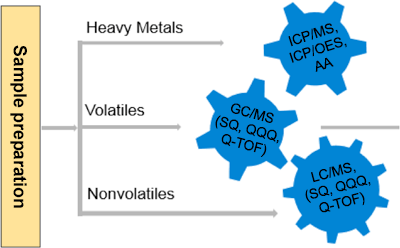
Figure 1. E&L workflow detects a wide variety of known and unknown organic/inorganic compounds that may be present in container closure systems.

Figure 2. Agilent ICP-MS and ICP-OES systems deliver accurate, repeatable results.
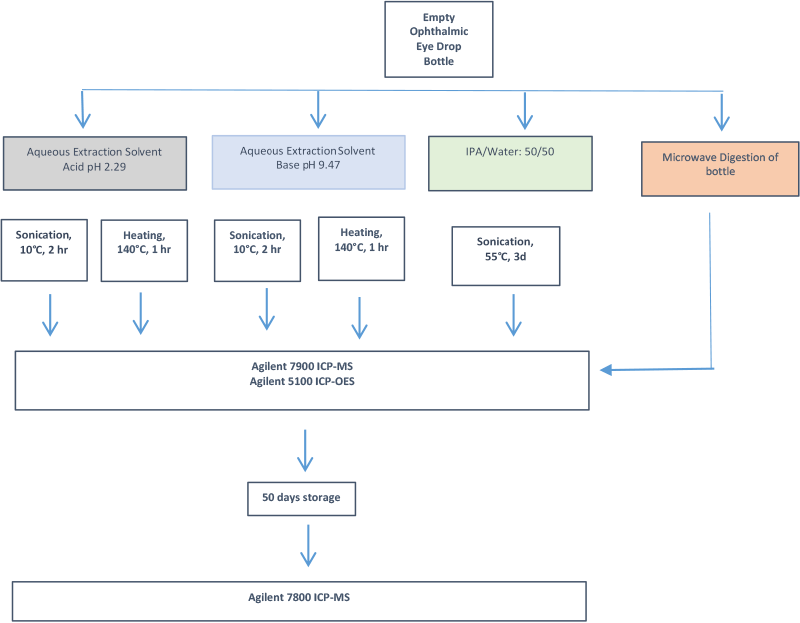
Figure 3. Workflow for this E&L study stresses the container with heating and shaking in various solvents, as well as extended storage.
Accurate detection and quantification of E&Ls requires highly specific and sensitive analytical methods that include LC/MS, GC/MS, and ICP-MS (Figure 1). This article describes the ICP-MS/ICP-OES workflow for elemental analysis.
Detection of E&Ls as elemental impurities requires reliable ICP-MS and ICP-OES
The United States Pharmacopeia (USP) is developing new General Chapters for the determination of elemental impurities in pharmaceutical products and ingredients. USP<232> defines the analyte limits, while USP<233> describes sample preparation options. It recommends the use of modern instrumentation such as multi-element ICP-MS and ICP-OES (Figure 2). Analytical equipment qualification under USP<233> is based on performance testing, and includes requirements to demonstrate accuracy, repeatability, and unequivocal identification of analytes.
Experimental design simulates storage conditions
For E&L analysis, it is important to predict realistic post-production stresses caused by transportation, marketplace storage, and at-home storage of the drug. For example, heating, physical shaking, and extended storage are aggressive stressors that can lead to decomposition of the container material and release of extractables into the drug product.
In a recent study on an ophthalmic drug packaging material, we simulated these concerns via treatment of plastic bottles with extraction solvents of varying pH and polarity. Some tests incorporated high temperature, sonication, and/or extended storage. To verify that the packaging material was indeed the source of detected contaminants, the container was completely microwave digested and analyzed. Finally, samples were stored for 50 days and re-measured to determine changes to the toxicity profile. These sample treatments are summarized in the workflow diagram in Figure 3.
Rapid results with difficult sample matrices
For this test, either ICP-OES or ICP-MS systems can be used. For our study, both instruments were utilized to perform elemental analysis of E&Ls.
The Agilent 5100 Synchronous Vertical Dual View (SVDV) ICP-OES, equipped with a Dichroic Spectral Combiner (DSC), enables a single reading that spans the entire wavelength range—utilizing both axial and radial view emissions. The vertically-oriented torch and solid-state RF system (27 MHz) stabilize plasma delivery, for the robust analysis of the high-organic samples of an E&L experiment. The SVDV mode is beneficial for an E&L study, because both trace-level and major elements are of interest.
The Agilent 7800 and 7900 ICP-MS offer exceptional matrix tolerance, to enable routine measurements at 25 percent total dissolved solids. This capability is especially useful for analyses of pharmaceutical matrices such as saline solution. The wide, 10-orders-of-magnitude dynamic range also permits simultaneous measurement of both trace and percent-level concentrations. For the lowest limits of detection and widest linear calibrations, ICP-MS is the ideal technique, especially for parenteral and inhalation drug products, where the required detection limits are much lower than for oral medicines.
Advanced tools for data mining and statistical analysis
Agilent MassHunter software provides an intuitive interface for data processing and review–from calibration curves to dilution factors to quality controls. On both quadrupole ICP-MS systems, the USP preset method in the MassHunter software is programmed to reduce setup time for USP analyses.
Agilent Mass Profiler Professional (MPP) is a chemometric tool that provides the power to import, mine, compute, relate, and visually interpret multiple data sets. The software performs a variety of statistical analyses that include principal component analysis (PCA) loadings, fold-change, box-whisker plots, and Venn diagrams. The data workups can be readily pivoted by sample parameter.
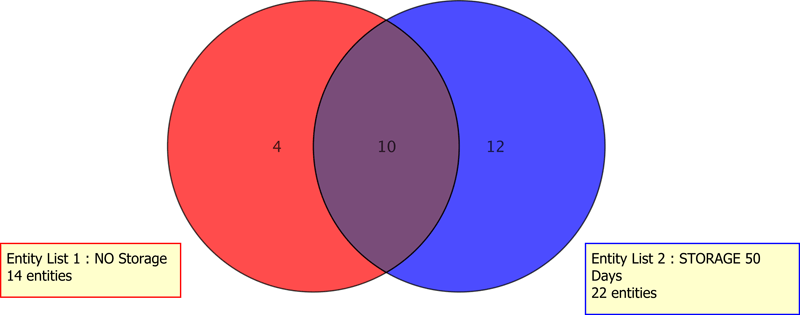
Figure 4. Both conserved and unique contaminants depended on the extraction condition or storage.
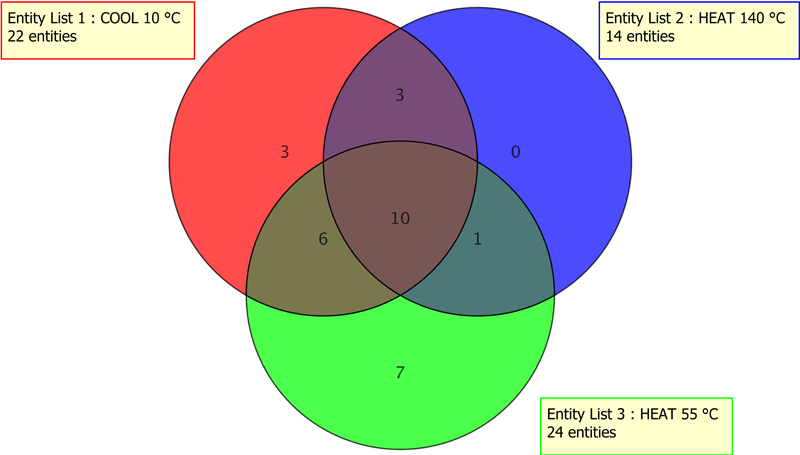
Figure 5. A single contaminant was detected in both of the heated samples (140 °C and 55 °C), as represented by the overlap regions of the blue and green circles.
In the ophthalmic drug E&L study, numerous stressors were tested: solvent, temperature, sonication, treatment duration, and storage. Venn diagrams (Figures 4 and 5) were rapidly generated to compare the individual effects of heat treatment, storage treatment, and solvent treatment on the elemental profiles. This level of data work-up would be tedious if not impossible without the readily available computations using Agilent MPP.
Accurately detect and quantify E&Ls
The analysis of extractable and leachable elemental impurities in pharmaceuticals is of vital importance. This article has examined workflows and system configurations for detection of E&Ls. To discover more about the analysis of elemental impurities in pharmaceutical products, explore our primer 5991-0436EN.
For an even wider range of information on the evolving area of E&L analysis, visit our resource page on that topic. Then contact your Agilent representative for further information.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
Article Directory – January 2017
All articles in this issue
-
 Best practices for food safety laboratories: highlights from inaugural Agilent—AOAC workshop
Best practices for food safety laboratories: highlights from inaugural Agilent—AOAC workshop -
 ICP-MS and ICP-OES methodology enables comprehensive analysis and quantification of elemental extractables and leachables
ICP-MS and ICP-OES methodology enables comprehensive analysis and quantification of elemental extractables and leachables -
 Ask the Expert: Is there a simple, cost-effective alternative to traditional manual operation while improving precision in GC analysis?
Ask the Expert: Is there a simple, cost-effective alternative to traditional manual operation while improving precision in GC analysis? -
 Case study: Agilent customer collaboration delivers robust LC-QQQ method to quantify vitamin B12 absorption
Case study: Agilent customer collaboration delivers robust LC-QQQ method to quantify vitamin B12 absorption -
 Accelerate analysis of mineral oil (MOSH/MOAH) with ready-to-go online LC-GC/FID coupling
Accelerate analysis of mineral oil (MOSH/MOAH) with ready-to-go online LC-GC/FID coupling -
 Preconfigured GC/MS/MS analyzer puts you on the fast track to pesticide analysis
Preconfigured GC/MS/MS analyzer puts you on the fast track to pesticide analysis -
 Tip: Getting the best value from your UHPLC analyses
Tip: Getting the best value from your UHPLC analyses
Figure 1

E&L workflow detects a wide variety of known and unknown organic/inorganic compounds that may be present in container closure systems.
Figure 2

Agilent ICP-MS and ICP-OES systems deliver accurate, repeatable results.
Figure 3

Workflow for this E&L study stresses the container with heating and shaking in various solvents, as well as extended storage.
Figure 4

Both conserved and unique contaminants depended on the extraction condition or storage.
Figure 5

A single contaminant was detected in both of the heated samples (140 °C and 55 °C), as represented by the overlap regions of the blue and green circles.