Access Agilent eNewsletter July 2016
Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research
Dr. Mustafa Kansiz and Dr. Carolina Livi, Agilent FTIR Microscopy & Bioinformatics Scientists
The era of high throughput Omics technologies has provided vast data sets of information. Big data projects have now also moved into the single cell domain and the importance of understanding the spatial differences in tissues and the cell type of specific profiles is clear.
Fourier Transform Infrared (FTIR) chemical imaging has been an important tool in many facets of analytical chemistry for almost 15 years and in recent times has found important uses in life science research. FTIR imaging uniquely provides a rapid 2D chemical snapshot of the sample, spatially resolved to a few microns across a large field of view.
FTIR chemical imaging is being used by many researchers to study the most common age-related diseases: cancer and neurodegeneration. Here we highlight work by Profs. Klaus Gerwert, Peter Gardner and Kathleen Gough who specialize in FTIR imaging in biomedical research.
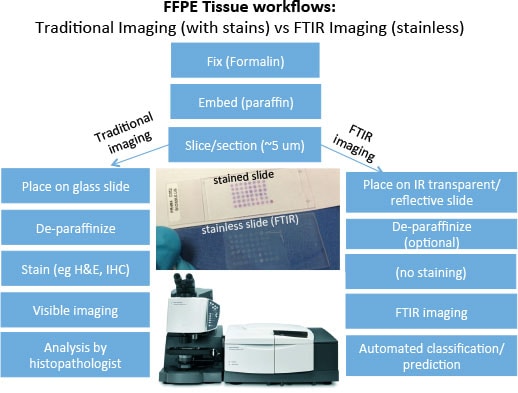
Figure 1. FTIR imaging workflows overlap with existing IHC pathology methods.

Figure 2. The first Random Forest (RF) detects different tissue types and pathological regions (left). The spectra of tumorous regions were transferred to the second RF, which determines the grading of the cancer cells. H&E stained sample of grade 2 tumor overlaid with IR spectral image. The three colors red, green and blue cover again the three grades in ascending order (right).
With an aging global population, research into human health, particularly cancer and neurological diseases continues to grow. While traditional genomic, proteomic and metabolomic techniques play a key role, FTIR imaging is emerging as a key supporting analytical tool. This is due to its unique ability to provide rapid micro scale spatially resolved macromolecular chemical information.
Analysis of Formalin Fixed Paraffin Embedded (FFPE) tissues is a staple of disease research. Disease tissues are often imaged with Hematoxylin and Eosin (H&E), ImmunoHistoChemical (IHC) and various other stains. These stains provide targeted spatial molecular information to highlight particular anatomical structures or other disease indicating features. Such stains are typically assessed and interpreted under a visible light microscope by a trained histopathologist. However this interpretation is subjective and often there is considerable inter-observer variability between different practitioners. Applications on FFPE samples, range from tissue segmentation to disease grading research and have been conducted on various disease tissues such as a prostate, breast, colon and lung cancers.
FTIR imaging provides a chemical image of the sample nondestructively and without the need for stains. With the appropriate calibration and machine learning algorithms, various tissue types and diseased states can be determined in an automated fashion. This automation removes the human subjectivity in classification and provides a more quantitative assessment. Furthermore, as the technique fits within existing sample preparation protocols (Figure 1), its utilization alongside traditional staining techniques integrates seamlessly with pathology workflows. The application of FTIR Imaging for histopathology is now being referred to as “Spectral HistoPathology (SHP)”.
A recent publication by Prof. Gerwert and colleagues [1] demonstrated a two stage prediction scheme (by a “random forest” ” mathematical calibration model) using FTIR Imaging. In this scheme, colon cancer tissues are first classified into anatomical type and then with a separate classifier (model), tumorous regions are graded (see Figure 2). This classification is all accomplished without any staining and human interaction or assessment in a fully automated manner. This approach uses the inherent chemical information and contrast of the sample that is detected and visualized using FTIR Imaging.

Figure 3. H&E visible image of TMA with an expanded view of the two cores used for constructing the histology spectral database (a), False color classification image of breast TMA FT-IR chemical image, where green = epithelium, purple = stroma, red = blood, and orange = necrosis (b). The expanded cores were used for building the histology model. Each core has a diameter of 1 mm and is represented by ca. 26000 IR spectra. Receiver operator curve (ROC) for the Random Forest classifier output for the independent test data set (c). Classification results from independent samples test set presented.

Figure 4. Distribution of both lipid and creatine in control and transegenic mouse brains as a function of age. Transgenic mice exhibit higher concentrations of creatine, in particular at an earlier age. Right, representative FTIR spectra of brain (gray matter), Creatine within the brain (a mixture of pure creatine and brain) and pure creatine.
Gardner and colleagues [2] also recently demonstrated an automated high-throughput assessment of prostate biopsy tissue using FTIR imaging and large Tissue Micro Array (TMA) samples. This method could accurately predict (97–100% accuracy) various important tissue structures; such as epithelium, smooth muscle, lymphocytes, blood, and necrotic regions (see Figure 3). This work also pioneered FTIR image collection on glass substrates, which are traditionally thought to be not suitable. They were able to distinguish key tissue types using only the high wavenumber spectral region, which is the only spectral region that is transmissive through glass.
These results are particularly encouraging and directly related to the potential of using FTIR imaging as a tool for the quality control of tissues. FTIR imaging ensures that the right tissue types are present before Omics studies analyzing nucleic acids (DNA/RNA), proteins or metabolites are conducted.
Applications looking for cellular deposits in brain tissues (both fixed and unfixed) have also been reported and are under active investigation. Neuroscience applications include Alzheimer’s disease studies with FTIR Imaging being able to provide cellular and subcellular chemical images. FTIR Imaging can distinguish between plaques, lipids and creatine formation and variety of other tissue types as well as research into the brain tumor progression.
Prof. Gough, a leading neuroscience researcher using FTIR imaging [3], studied transgenic mouse models of Alzheimer’s disease to probe the chemical distribution of brain tissues, particularly as they relate to plaque formation and their association with creatine and lipids (see Figure 4). FTIR imaging provided direct chemical information on the distribution and association of various components of what is otherwise a difficult to stain system.
Advances in FTIR imaging continue in areas such as optics for ultrawide field and ultra-high magnification imaging together with advances in “big data” analysis which is facilitated by increased computing capacity of modern PCs with optimized data collection and processing protocols. These combined improvements, together with recent key scientific publications demonstrate that FTIR imaging has advanced to a point where it is practical and feasible to adopt as part of life science workflows where it can improve sample quality assessment and provides a new tool with which to study various biological systems.
References
- C. Kuepper, F. Großerüschkamp, A. Kallenbach-Thieltges, A. Mosig, A. Tannapfel and K. Gerwer. “Label-free Classification of Colon Cancer Grading using Infrared Spectral Histopathology.” Faraday Discussions, DOI: 10.1039/C5FD00157A (2015)
- Paul Bassan, Joe Mellor, Jonathan Shapiro, Kaye J. Williams, Michael Lisanti and Peter Gardner. “Transmission FT-IR Chemical Imaging on Glass Substrates: Applications in Infrared Spectral Histopathology.” Analytical Chemistry, 86(3), 1648–1653, (2014).
- Alexandra Kuzyk, Marzena Kastyak, Veena Agrawal, Meghan Gallant, Gajjeraman Sivakumar, Margaret Rak, Marc R. Del Bigio, David Westaway, Robert Julian, Kathleen M. Gough. “Association among Amyloid Plaque, Lipid, and Creatine in Hippocampus of TgCRND8 Mouse Model for Alzheimer Disease.” The Journal of Biological Chemistry. 285 (41), 31202–31207 (2010).
For Research Use Only. Not for use in diagnostic procedures.
This information is subject to change without notice.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application
Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds
Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds Tip: Using enzymes in dissolution testing of gelatin capsules
Tip: Using enzymes in dissolution testing of gelatin capsules InfinityLab Poroshell 120 columns maximize LC workflow efficiency
InfinityLab Poroshell 120 columns maximize LC workflow efficiency Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research
Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research New LC/TQ database and method for central carbon metabolites
New LC/TQ database and method for central carbon metabolites Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication
Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns
Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns Structural elucidation of in-process impurities using high resolution LC/MS/MS
Structural elucidation of in-process impurities using high resolution LC/MS/MS Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS
Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Figure 1

FTIR imaging workflows overlap with existing IHC pathology methods.
Figure 2

The first Random Forest (RF) detects different tissue types and pathological regions (left). The spectra of tumorous regions were transferred to the second RF, which determines the grading of the cancer cells. H&E stained sample of grade 2 tumor overlaid with IR spectral image. The three colors red, green and blue cover again the three grades in ascending order (right). (Reproduced by permission of The Royal Society of Chemistry)
Figure 3

H&E visible image of TMA with an expanded view of the two cores used for constructing the histology spectral database (a), False color classification image of breast TMA FT-IR chemical image, where green = epithelium, purple = stroma, red = blood, and orange = necrosis (b). The expanded cores were used for building the histology model. Each core has a diameter of 1 mm and is represented by ca. 26000 IR spectra. Receiver operator curve (ROC) for the Random Forest classifier output for the independent test data set (c). Classification results from independent samples test set presented. ("Reprinted and adapted with permission from Paul Bassan, Joe Mellor, Jonathan Shapiro, Kaye J. Williams, Michael Lisanti and Peter Gardner, Transmission FT-IR Chemical Imaging on Glass Substrates: Applications in Infrared Spectral Histopathology Anal. Chem. 86(3) (2014) 1648–1653). Copyright (2016) American Chemical Society)
Figure 4

Distribution of both lipid and creatine in control and transegenic mouse brains as a function of age. Transgenic mice exhibit higher concentrations of creatine, in particular at an earlier age. Right, representative FTIR spectra of brain (gray matter), Creatine within the brain (a mixture of pure creatine and brain) and pure creatine.