Agilent Labs' Len Cutler Leaves a Lasting Legacy
|
|
February 27, 2004
Research resulting from a collaboration between Agilent and Stanford's Reynolds Center is finding the genetic markers that could signal the onset of heart disease When two leading research centers in the world successfully pair their expertise, the results are astonishing. For the past three years, Agilent Laboratories, the central research facility of Agilent Technologies, has been involved in a collaborative effort with the Donald W. Reynolds Cardiovascular Clinical Research Center at Stanford University to study and decipher the expression of genes that are linked to heart disease, the leading cause of death in the United States. The research combines Agilent's DNA microarray technology and Agilent Labs' advanced computational capabilities with the expertise of Stanford's top-notch biologists to not only identify the genes linked to heart disease but to be able to use that information to understand which molecular pathways mediate the primary disease process in the blood vessel wall. The importance of this research collaboration stems from the statistics. Heart disease accounts for 40 percent of all deaths in this country and the numbers are on the rise. Each year more than 2,600 individuals die of the disease each day, or almost 1 million per year, more than the next seven leading causes of death combined. Astonishingly, about one-third of the people with heart disease die before they can even be diagnosed. "There is presently no way of predicting and no mechanism for screening people to determine who needs to be treated," says Tom Quertermous, MD, Director of the Division of Cardiovascular Medicine at the Reynolds Center. A Heart to Heart Collaboration
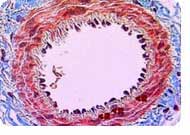
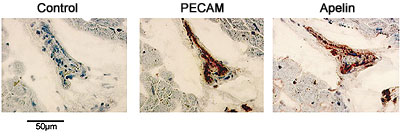
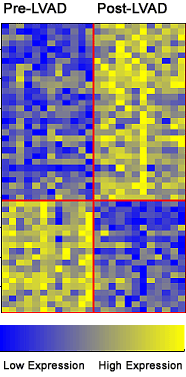
On the flip side, the Reynolds Center gives Agilent Labs' researchers access to its experts in the field of cardiovascular disease, as well as permission to explore data sets that include human patient samples and clinical annotation that they would otherwise not be able to access. "There is great synergy derived from our highly interactive relationship with the Reynolds Center scientists," says Laurakay Bruhn, project manager in the Molecular Diagnostics Department at Agilent Labs. "It greatly increases our ability to develop novel and effective prototype computational tools that allow us to stay at the leading-edge of methods for understanding and interpreting complex data sets like those derived from the microarray-based expression profiling studies." The initial and long-term focus of the collaboration has been on the most common form of heart disease -- atherosclerotic coronary artery disease, which is characterized by the gradual build-up of primarily fat and cholesterol on the inside walls of the coronary arteries that supply the heart muscle with the oxygen and nutrients it needs. "This probably represents one of the largest efforts in the world that involves trying to write the book on gene-expression patterns that correlate with heart disease," says Quertermous. He says that the goal is to implement a systematic strategy to isolate the genes that are regulated by pathophysiologically relevant stimuli in cells involving the vascular wall and then identify those genes whose in vivo expression correlates with specific vascular disease states. The Newly Discovered Gene Researchers compared the gene-expression patterns in the left ventricles of 12 patients obtained before and after they had a left ventricular assist device (LVAD) placed in their hearts for different periods of time. The researchers found a higher concentration of APJ in heart tissue after the placement of the LVAD. Further study found that apelin, a molecule that activates APJ, is localized primarily in the endothelium in the coronary arteries and increases in the blood levels of patients with left ventricular dysfunction. In this study, the apelin-APJ signaling pathway has been determined for the very first time to play a significant clinical role in the mediation of cardiovascular control.
"When we looked at the average of 12 pre-LVAD samples and compared them to the 12 post-LVAD samples, there weren't many genes that consistently discriminated between the two states," says Bruhn. "However, when we looked at consistent per patient differences in the pre- and post-LVAD samples, we were able to observe more statistically significant changes in gene expression." "This study was a good example of how this collaboration helped us to develop new methodology and new ways to analyze data," says Anya Tsalenko, senior scientist at Agilent Labs. Quertermous' group is pursuing parallel studies in animal models, primarily using genetic mouse models to do comparison studies between mouse and human tissues. "If we can manipulate the genes in mice that we already understand in humans, we can compare two disease processes," says Quertermous. "The samples collected from the vessels were very complicated mixtures of different cells," says Tsalenko. "And they were all coming from diseased patients so the differences in expression patterns were very subtle. Therefore, it was very important to figure out the best way to statistically examine the differences." In order to analyze this data set, Agilent Labs researchers developed a tool for the systematic analysis of groups of differentially expressed genes. From this study, they were able to conclude that inflammation and inflammatory response genes play an important role in the processes occurring in diseased blood vessels.
Another important study in the collaboration is examining the differences in gene expression of the veins and arteries. "People pay much more attention to studying arteries than veins, because veins rarely develop atherosclerosis whereas arteries do," say David Deng, a molecular biologist at Agilent Labs also working in the project. "By comparing the differences in gene expression between the two vessels, we can find new genes that are related to the formation of atherosclerosis. In fact, we did find a set of genes in cultured vascular cells from veins and arteries that have marked differences in response to atherosclerotic stimuli." The groups also want to determine why certain grafted blood vessels have different long-term outcomes following heart bypass surgery. One of the biggest difficulties encountered in human cardiovascular studies is dealing with the small number of samples that are available. When probing human data, there are large variations in gene-expression patterns, and confounding factors, such as age and sex that are difficult to control especially when small sample numbers are involved. "What Agilent Labs has brought to the table is the very finest microarray technology in the world," says Quertermous. "I don't have to ever wonder if the data is real or not -- I know it's the best. They have also produced new ways of looking at the data that are extremely powerful." Making A Difference Bruhn says that long-term research interests for Agilent Labs include understanding what roles multiplexed molecular profiling like that enabled by microarray-based technologies can play as a diagnostic tool in a clinical setting. "This collaboration with the Reynolds Center enables us to begin to explore strategies for using multiplexed molecular profiling in molecular diagnostic procedures related to cardiovascular disease." Related InformationAgilent News Feature: Agilent's whole human genome microarray speeds disease research through industry-leading sensitivity and open platform (Feb. 3, 2004)
|