Fragment Analyzer Rinse Buffer Update

Rinse buffers are required during normal instrument operation to clean the capillary tips and inlet electrodes prior to sample injection. Currently, Fragment Analyzer reagent kits use two different rinse buffers, 0.25x TE and 0.6x TE, the only difference being the final salt concentration.
Agilent is continuously looking for ways to improve the use of our products and exceed customer expectations, so with that in mind, we have successfully tested the use of only the 0.25x TE Rinse Buffer in all parallel CE reagent kits. This will provide our customers better kit flexibility and a more streamlined instrument operation.
Starting on November 1st 2019, the TE Rinse Buffer supplied with the kit part numbers listed below will change from 0.6x TE Rinse Buffer to 0.25x TE Rinse Buffer:
- DNF-473-0500 and DNF-473-1000
- Agilent NGS Fragment kit (1-6000bp)
- View validation test
- DNF-479-0500-OB
- Agilent NGS Fragment kit (35-6000bp)
- View validation test
- DNF-487-0500 and DNF-487-1000
- Agilent Genomic DNA kit
- View validation test
- DNF-492-0500 and DNF-492-1000
- Agilent Large Fragment kit
- View validation test
For additional information, please see the FAQs section.
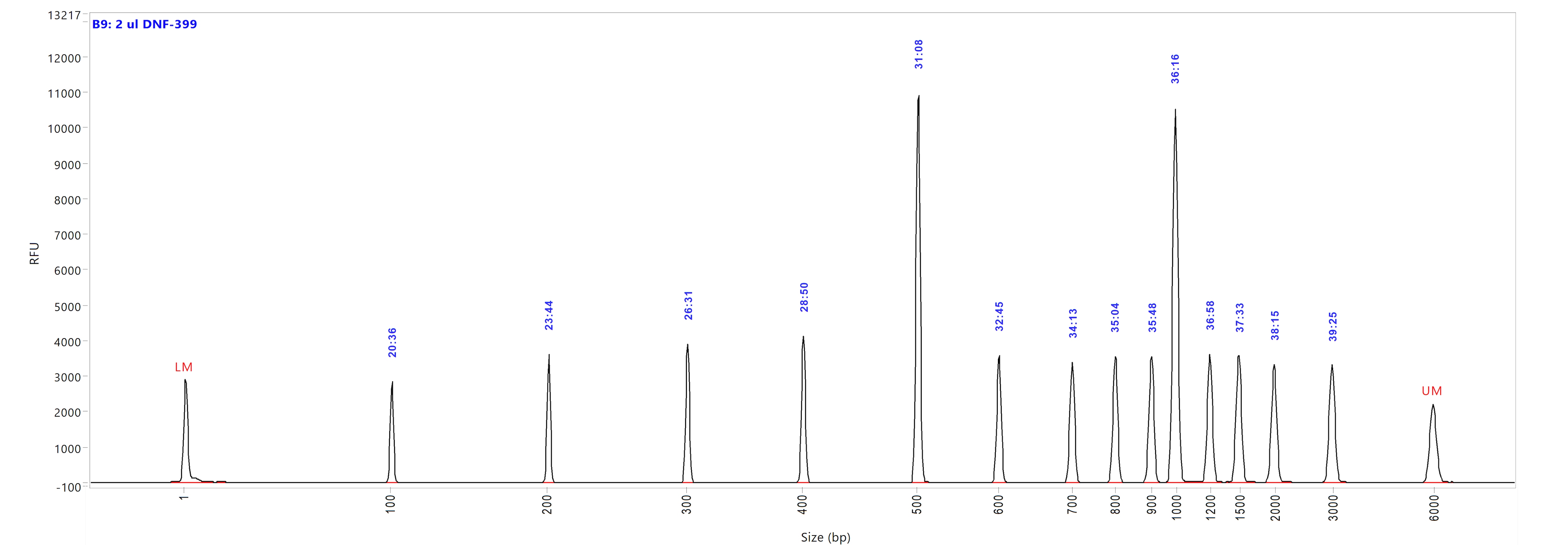
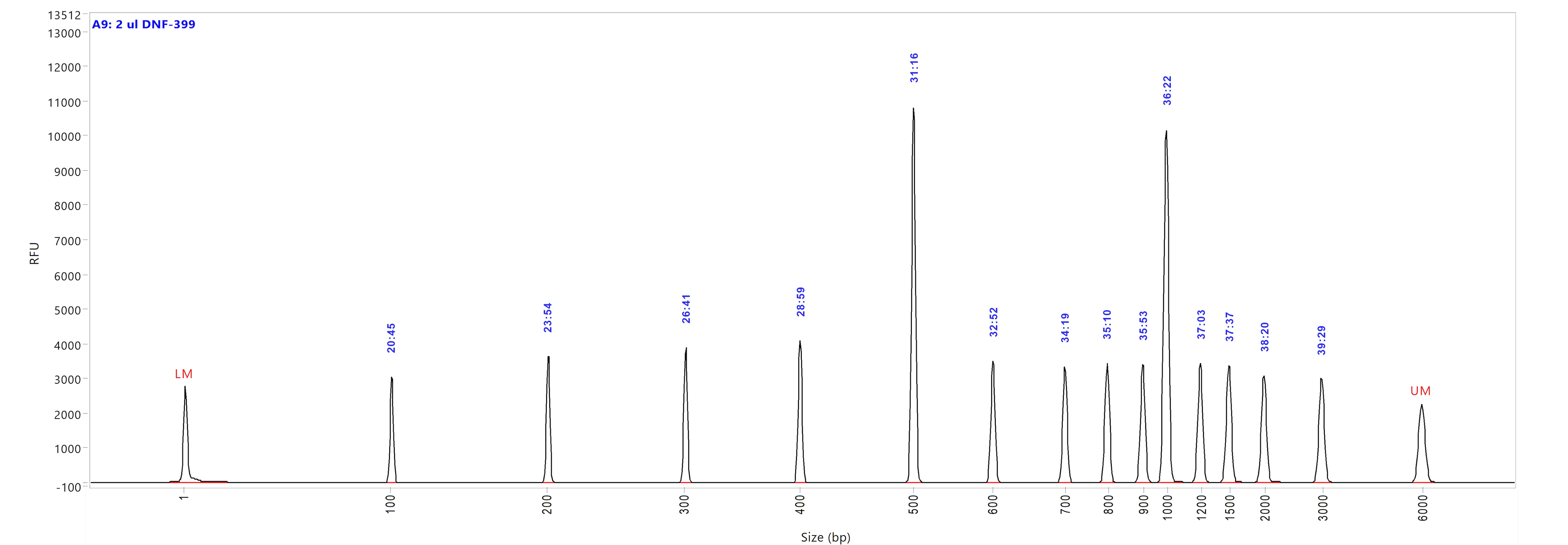
Run performed using the NGS DNA Ladder (p/n DNF-399-U100). The electropherogram images show the peak distribution sizing in bp and the peak migration time (in blue).
Image A shows ladder results using 0.25x TE Rinse Buffer prior to the sample injection step.
Image B shows ladder results using 0.6x TE Rinse Buffer prior to the sample injection step.
Both test results show close correlation for the peak size as well as similar migration time of the peaks.
NGS Fragment Kit (35-6000bp)
Run performed using the NGS DNA Ladder (p/n DNF-479-U100). The electropherogram images show the peak distribution sizing in bp and the peak migration time (in blue).
Image A shows ladder results using 0.25x TE Rinse Buffer prior to the sample injection step.
Image B shows ladder results using 0.6x TE Rinse Buffer prior to the sample injection step.
Both test results show close correlation for the peak size as well as similar migration time of the peaks.
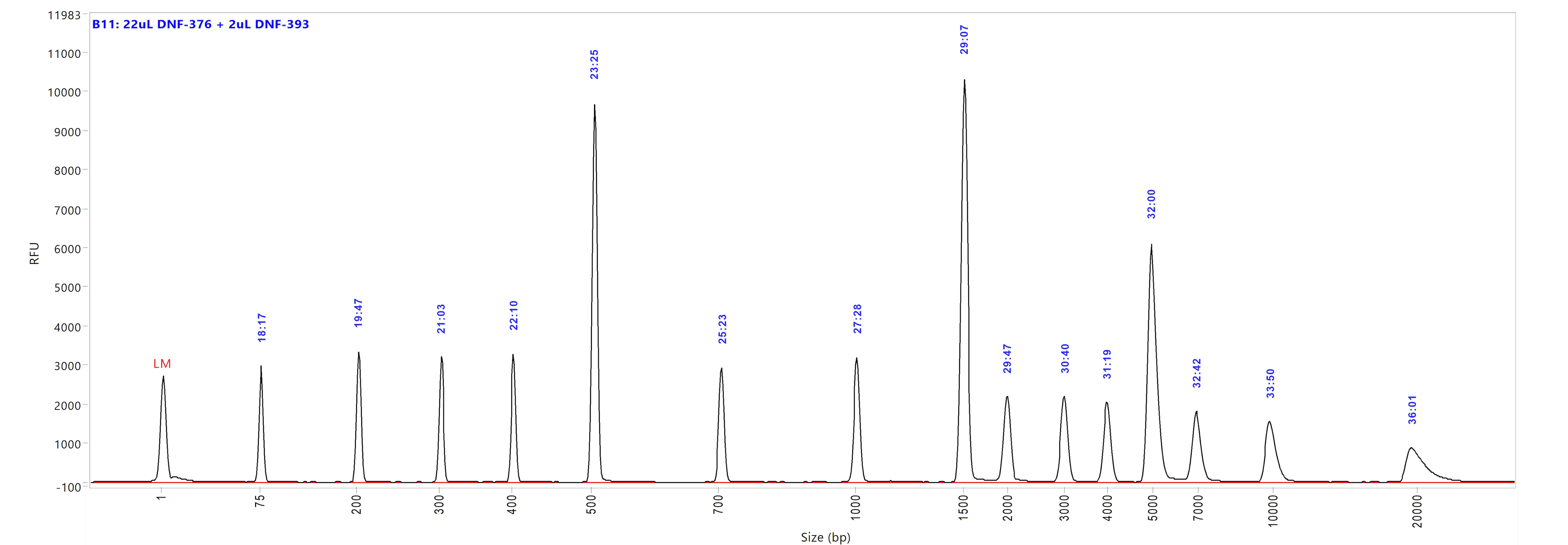
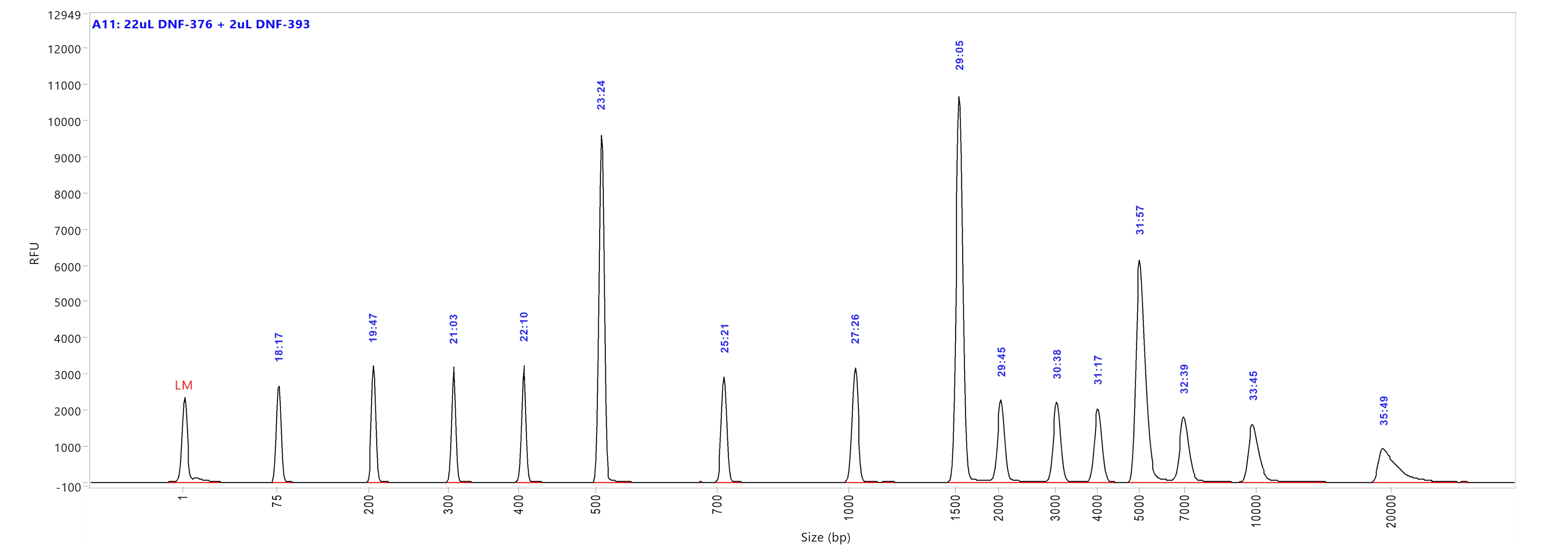
Genomic DNA kit
Run performed using the Genomic DNA Ladder (p/n DNF-393-U100). The electropherogram images show the peak distribution sizing in bp and the peak migration time (in blue).
Image A shows ladder results using 0.25x TE Rinse Buffer prior to the sample injection step.
Image B shows ladder results using 0.6x TE Rinse Buffer prior to the sample injection step.
Both test results show close correlation for the peak size as well as similar migration time of the peaks.
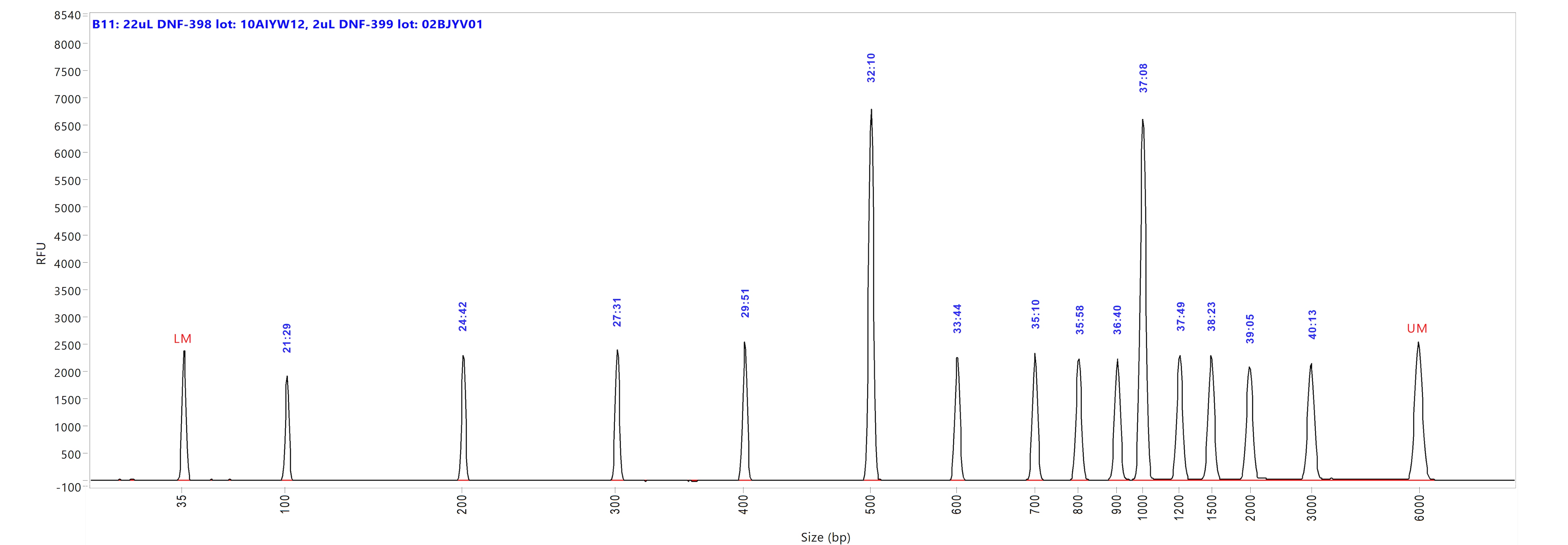
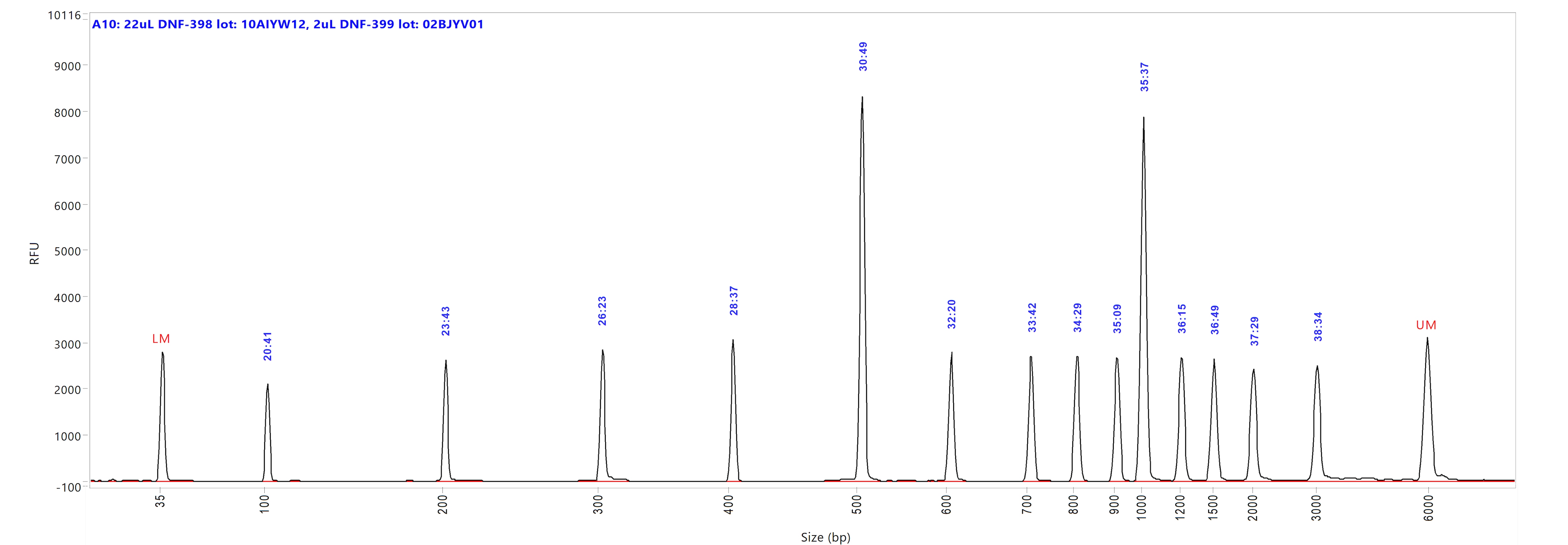
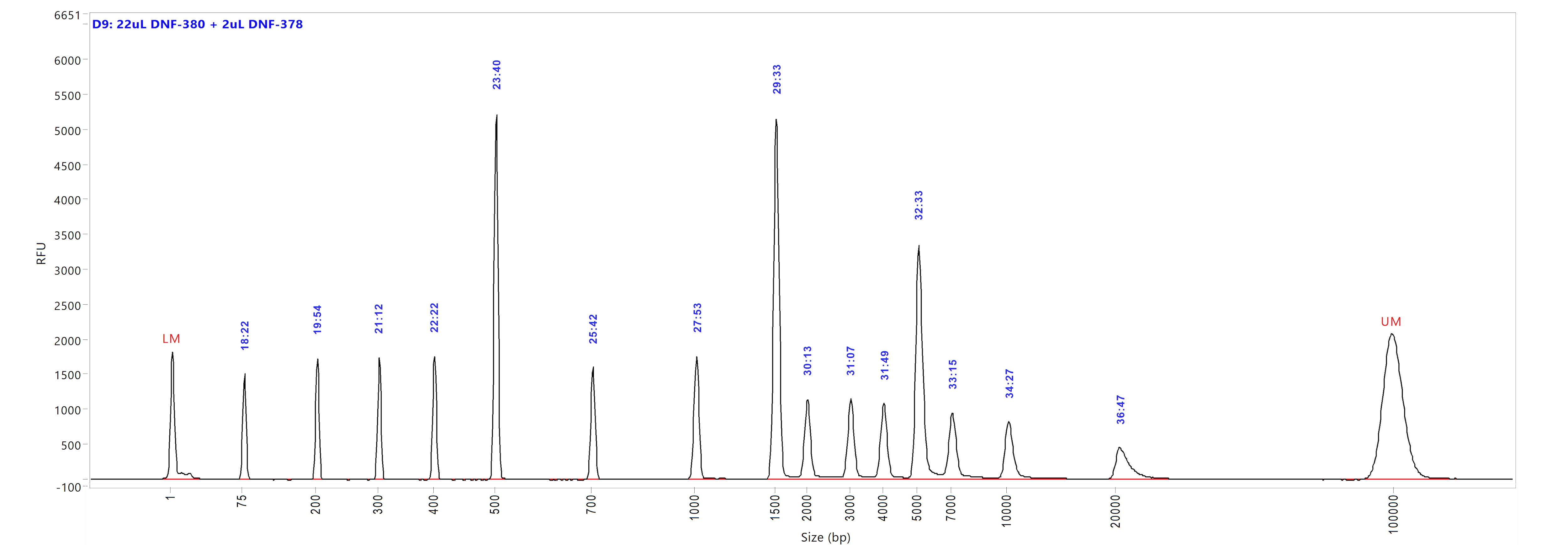
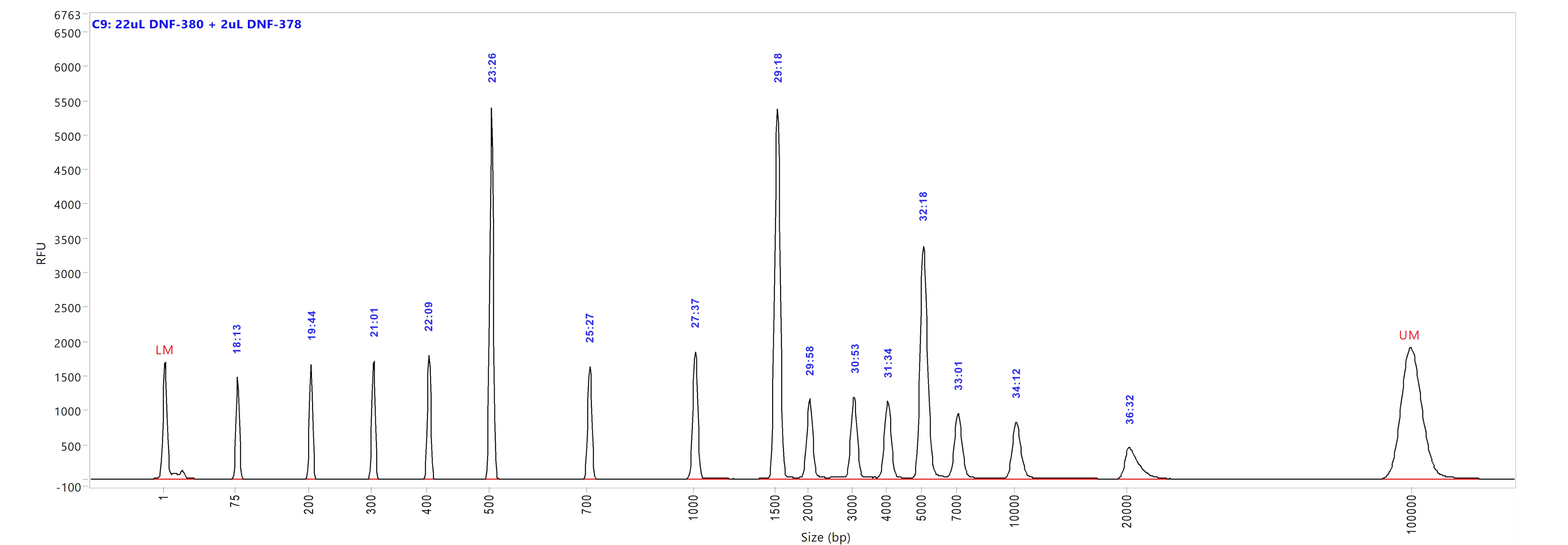
Large Fragment kit
Run performed using the Large Fragment DNA Ladder (p/n DNF-393-U100). The electropherogram images show the peak distribution sizing in bp and the peak migration time (in blue).
Image A shows ladder results using 0.25x TE Rinse Buffer prior to the sample injection step.
Image B shows ladder results using 0.6x TE Rinse Buffer prior to the sample injection step.
Both test results show close correlation for the peak size as well as similar migration time of the peaks.
Q: Why do I need to use the rinse buffer?
A: The rinse buffer is used to clean the capillary tips after the gel fill step and before the sample injection.
Q: Should I expect to see any change in my data when switching from the 0.6x TE Rinse Buffer to the 0.25x TE Rinse Buffer?
A: No. You should not expect any changes to your data when switching to the 0.25x TE Rinse Buffer.
Q: What is storage condition to the 0.25x TE Rinse Buffer?
A: The 0.25x TE Rinse Buffer should be store at 4°C which is the same storage temperature as the 0.6x TE Rinse Buffer
Q: Is there any change to the protocol or to the separation method when I switch from using the 0.6x TE Rinse Buffer to 0.25x TE Rinse Buffer?
A: No, there are no protocol changes. You will continue to prepare a fresh sample plate filled with 200 μL/well. The separation method are the same as before.
Q: Is the volume of 0.25x TE Rinse Buffer bottle provided the same as the 0.6x TE Rinse Buffer?
A: Yes. The volume of the 0.25x TE Rinse Buffer is the same as the 0.6x TE Rinse Buffer
Q: If I want to do my own validation study using the 0.25x TE Rinse Buffer can I purchase the 0.6x Rinse Buffer as standalone part?
A: Yes, the 0.6x Rinse Buffer will be available for sale as a standalone part (Part Number DNF-496-0125). This part will be available until October 2020.
Q: Are there any changes on the expiration time of the 0.6x TE Rinse Buffer compared to the 0.25x TE Rinse Buffer?
A: No, the shelf life will not change. All the kit components, including the 0.25x and the 0.6x TE Rinse Buffers have a minimum of 4 months guaranteed shelf life, but expiration time usually goes beyond 4 months.
Q: . How long prior to the change will Agilent notify customers about the change of the rinse buffer?
A: Agilent will notify customers for 3 months prior to implementing this change.
For Research Use Only. Not for use in diagnostic procedures.