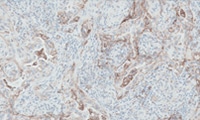
PD-L1 IHC 28-8 pharmDx Overview


- Broad Utility: Evidence based PD-L1 testing across multiple cancer types
- Clinically relevant PD-L1 results linked to clinical outcomes
- Unrivaled quality and reliability
- Standardized and fully validated kit
- Integrate PD-L1 IHC 28-8 pharmDx without changing staining lab workflow
- Ready-to-use reagents and cell line controls optimized for Autostainer Link 48
- Pre-programmed, validated protocol
Esophageal squamous cell carcinoma (ESCC)
Click here to download Manual and Brochure
Gastric, gastroesophageal junction (GEJ) adenocarcinoma, or
fesophageal adenocarcinoma
Click here to download Manual and Brochure
Non-squamous non-small cell lung cancer (nsNSCLC)
Click here to download Manual and Brochure
Squamous cell carcinoma of the head and neck (SCCHN)
Click here to download Manual and Brochure
Muscle invasive urothelial carcinoma (MIUC)
Click here to download Manual and Brochure
Read about the interpretation of PD-L1 IHC 28-8 pharmDx staining results.
Interpretation of MIUC staining results
Interpretation of ESCC staining results
Interpretation of Gastric, GEJ, or esophageal adenocarcinoma staining results
Interpretation of nsNSCLC staining results
Interpretation of SCCHN staining results
PD-L1 IHC 28-8 pharmDx is a complete kit with reagents sufficient for 50 tests (50 slides incubated with primary antibody to PD-L1 and 50 slides incubated with the corresponding Negative Control Reagent) and 15 Control Slides for use on Autostainer Link 48.

- EnVision FLEX Target Retrieval Solution, Low pH, 50x
- Peroxidase-Blocking Reagent
- Monoclonal Rabbit Anti-PD-L1, Clone 28-8
- Negative Control Reagent
- Linker, Anti-Rabbit
- Visualization Reagent-HRP
- DAB+ Substrate Buffer
- DAB+ Chromogen
- DAB Enhancer
- Control Slides
|
Product |
Code |
|---|---|
|
SK005 |
|
|
Required but not included in the kit: Autostainer Link 48 EnVision FLEX Wash Buffer, 20x EnVision FLEX Hematoxylin (Link) PT Link PT Link Rinse Station |
AS480 K8007 K8008 PT100/PT200 PT109 |
Intended Use
For in vitro diagnostic use.
PD-L1 IHC 28-8 pharmDx is a qualitative immunohistochemical assay using Monoclonal Rabbit Anti-PD-L1, Clone 28-8 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-squamous non-small cell lung cancer (nsNSCLC), squamous cell carcinoma of the head and neck (SCCHN), urothelial carcinoma (UC), melanoma, gastric adenocarcinoma, gastroesophageal junction (GEJ) adenocarcinoma, and esophageal carcinoma tissues using EnVision FLEX visualization system on Autostainer Link 48.
PD-L1 protein expression in nsNSCLC, SCCHN, UC, muscle invasive urothelial carcinoma (MIUC), melanoma, and esophageal squamous cell carcinoma (ESCC) is determined by using % tumor cell expression, which is the percentage of evaluable tumor cells exhibiting partial or complete membrane staining at any intensity.
PD-L1 protein expression in gastric adenocarcinoma, GEJ adenocarcinoma, and esophageal adenocarcinoma is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
Companion Diagnostic Indications
|
Tumor Indication |
PD-L1 Expression Clinical |
Intended Use |
|
MIUC |
≥ 1% tumor cell expression |
PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying MIUC patients for treatment with OPDIVO® (nivolumab). |
|
ESCC |
≥ 1% tumor cell expression |
PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying ESCC patients for treatment with OPDIVO® (nivolumab) in combination with fluoropyrimidine and platinum-based chemotherapy or OPDIVO® (nivolumab) in combination with YERVOY® (ipilimumab). |
|
Gastric, GEJ, or Esophageal Adenocarcinoma |
CPS ≥ 5 |
PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying gastric, gastroesophageal junction, or esophageal adenocarcinoma patients for treatment with OPDIVO® (nivolumab) in combination with fluoropyrimidine and platinum-based chemotherapy. |
PD-L1 expression (≥ 1% or ≥ 5% or ≥ 10% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in non-squamous NSCLC (nsNSCLC) may be associated with enhanced survival from OPDIVO® (nivolumab).
PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in SCCHN may be associated with enhanced survival from OPDIVO® (nivolumab).
PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in urothelial carcinoma may be associated with enhanced response rate from OPDIVO® (nivolumab).
PD-L1 expression (≥ 1% or ≥ 5% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in melanoma may be used as an aid in the assessment of patients for whom OPDIVO® (nivolumab) and YERVOY® (ipilimumab) combination treatment is being considered.
See the local OPDIVO® and YERVOY® product labels for specific clinical circumstances guiding PD-L1 testing.
References
D70798
- PD-L1 IHC 28-8 pharmDx Instructions for Use
- CheckMate-274, CA209274
- CheckMate-648, CA209648
- CheckMate-649, CA209649
- CheckMate-057, CA209057
- CheckMate-141, CA209141
- CheckMate-275, CA209275
- CheckMate-067, CA209067