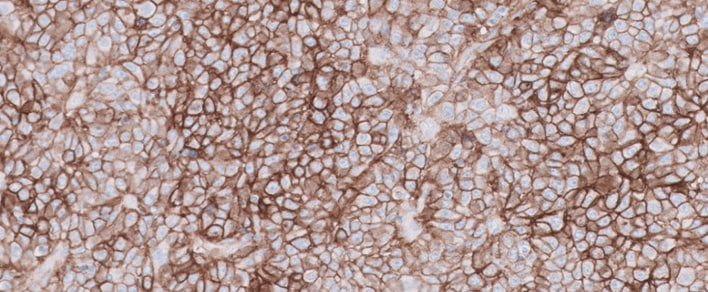
PD-L1 IHC 22C3 pharmDx Testing for TNBC

In TNBC, PD-L1 testing with PD-L1 IHC 22C3 pharmDx (SK006) can help identify patients for treatment with KEYTRUDA1,2
- In Europe, breast cancer is the most common type of cancer diagnosed in women,3 and approximately 15–20% of breast cancer diagnoses are TNBC.4 Patients with TNBC have significantly worse overall survival times than non-TNBC patients5
- PD-L1 testing provides a direct assessment of PD-L1 expression, which is a biomarker for response to anti-PD-1 therapy in TNBC1,2
- In the KEYNOTE-355 clinical trial, PD-L1 testing with PD-L1 IHC 22C3 pharmDx was used to assess PD-L1 expression in patients with previously untreated locally recurrent unresectable or metastatic TNBC, who had not received prior chemotherapy for metastatic disease*1,2
* Review the PD-L1 IHC 22C3 pharmDx Instructions for Use for more information regarding the KEYNOTE clinical trials
PD-L1 IHC 22C3 pharmDx was the only PD-L1 assay used in the KEYTRUDA KEYNOTE-355 clinical trial in TNBC1,2
PD-L1 IHC 22C3 pharmDx is CE-IVD–marked as the clinical trial‑proven companion diagnostic for KEYTRUDA1

† Chemotherapy: paclitaxel, nab-paclitaxel, or gemcitabine and carboplatin

When confidence in a PD-L1 test is critical, the ONE you choose is crucial
- The ONE PD-L1 assay used in KEYTRUDA clinical trials1,2
- The ONE PD-L1 assay first launched with KEYTRUDA in every indication that requires PD-L1 testing1,2
- The ONE PD-L1 assay trusted worldwide to test hundreds of thousands of patients for KEYTRUDA6


KEYTRUDA is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
References: 1. PD-L1 IHC 22C3 pharmDx [Instructions for Use]. Santa Clara, CA: Agilent Technologies, Inc.; 2021. 2. Keytruda [Summary of Product Characteristics]. European Medicines Agency; 2021. 3. World Health Organization. Global Cancer Observatory. https://gco.iarc.fr/today/data/factsheets/populations/908-europe-fact-sheets.pdf (accessed April 27, 2022). 4. Ismail-Khan R, Bui MM (2010) A review of triple-negative breast cancer. Cancer Control 17(3):173–176. 5. Li, X.; Yang, J.; Peng, L.; Sahin, A. A.; Huo, L.; Ward, K. C.; O’Regan, R.; Torres, M. A.; Meisel, J. L. Triple-Negative Breast Cancer Has Worse Overall Survival and Cause-Specific Survival than Non-Triple-Negative Breast Cancer. Breast Cancer Res Treat. 2016, 161(2), 279–287. 6. Data on file. Agilent Technologies, Inc.
For countries outside of the European Union, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
D67314_01