PD-L1 IHC 22C3 pharmDx Testing for NSCLC

In NSCLC, PD-L1 testing with PD-L1 IHC 22C3 pharmDx can help identify patients for first-line treatment with KEYTRUDA monotherapy1,2
- Lung cancer is the leading cause of cancer-related mortality in Europe,3 and NSCLC accounts for ~85% of all lung cancer cases4
- In 2017, KEYTRUDA became the first anti-PD-1 monotherapy EMA approved at first line for patients with metastatic NSCLC5
- PD-L1 testing provides a direct assessment of PD-L1 expression, which is a biomarker for response to anti-PD-1 therapy in NSCLC1,2
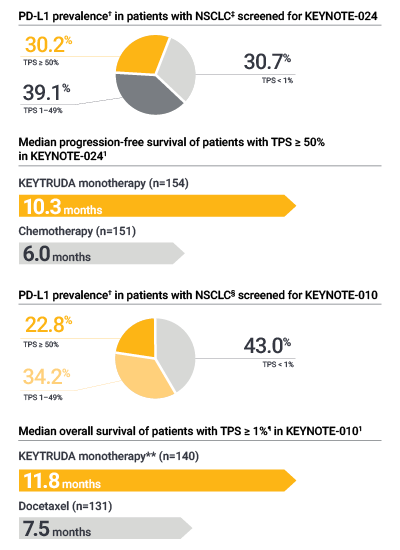
- PD-L1 testing with PD-L1 IHC 22C3 pharmDx was used to qualify patients with NSCLC for treatment with KEYTRUDA monotherapy in the KEYNOTE-024 and KEYNOTE-010* clinical trials1,2
The only PD-L1 assay used in the KEYTRUDA KEYNOTE-024 and KEYNOTE-010 clinical trials in NSCLC1,2
PD-L1 IHC 22C3 pharmDx is CE-IVD–marked as the clinical trial-proven companion diagnostic for KEYTRUDA monotherapy, and was used to assess PD-L1 expression and select patients for treatment in in KEYNOTE-024 and KEYNOTE-0101

* KEYNOTE-010 evaluated the safety and efficacy of KEYTRUDA in patients with advanced NSCLC previously treated with platinum-containing chemotherapy. Patients were screened for enrollment using a clinical-trial assay, † Merck & Co., data on file, ‡ Patients screened for enrollment in KEYNOTE-024 NSCLC, § Patients screened for enrollment in KEYNOTE-010 NSCLC using the clinical-trial assay, ¶ Patients tested with PD-L1 IHC 22C3 pharmDx, ** 2 mg/kg every 3 weeks. For efficacy data on the 10 mg/kg treatment arm, review the Instructions for Use
Note: PD-L1 testing with PD-L1 IHC 22C3 pharmDx was used to qualify patients with NSCLC for first-line treatment with KEYTRUDA monotherapy in the KEYNOTE-042 clinical trial. For more information on the KEYNOTE clinical trials, review the Instructions for Use.
When confidence in a PD-L1 test is critical, the ONE you choose is crucial
- The ONE PD-L1 assay used in KEYTRUDA clinical trials1,2
- The ONE PD-L1 assay first launched with KEYTRUDA in every indication that requires PD-L1 testing1,2
- The ONE PD-L1 assay trusted worldwide to test hundreds of thousands of patients for KEYTRUDA9


KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
References: 1. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2020. 2. Keytruda [Summary of Product Characteristics]. European Medicines Agency; 2020. 3. World Health Organization. Global Cancer Observatory. http://gco.iarc.fr/today/data/factsheets/populations/994-who-europe-region-euro-fact-sheets.pdf (accessed June 19, 2020). 4. Wood, R.; Taylor-Stokes, G.; Smith, F.; Chaib, C. The Humanistic Burden of Advanced Non-Small Cell Lung Cancer (NSCLC) in Europe: A Real-World Survey Linking Patient Clinical Factors to Patient and Caregiver Burden. Quality of Life Research 2019, 28 (7), 1849–1861. 5. European Commission approves KEYTRUDA® (pembrolizumab) for first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have high PD-L1 expression with no EGFR or ALK positive tumor mutations [news release]. Kenilworth, NJ; Merck & Co., Inc.; January 31, 2017. https://investors.merck.com/news/press-release-details/2017/European-Commission-Approves-KEYTRUDA-pembrolizumab-for-First-Line-Treatment-of-Patients-with-Metastatic-Non-Small-Cell-Lung-Cancer-NSCLC-Whose-Tumors-Have-High-PD-L1-Expression-with-No-EGFR-or-ALK-Positive-Tumor-Mutations/default.aspx. Accessed June 19, 2020. 6. Brahmer, J. R.; Govindan, R.; Anders, R. A.; Antonia, S. J.; Sagorsky, S.; Davies, M. J.; Dubinett, S. M.; Ferris, A.; Gandhi, L.; Garon, E. B.; et al. The Society for Immunotherapy of Cancer Consensus Statement on Immunotherapy for the Treatment of Non-Small Cell Lung Cancer (NSCLC). Journal for ImmunoTherapy of Cancer 2018, 6 (1). 7. Hanna, N.; Johnson, D.; Temin, S.; Masters, G. Systemic Therapy for Stage IV Non–Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. Journal of Oncology Practice 2017, 13 (12), 832–837. 8. Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.; Faivre-Finn, C.; Mok, T.; Reck, M.; Schil, P. V.; Hellmann, M.; et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2018, 29, iv192–iv237. 9. Data on file. Agilent Technologies, Inc.
For countries outside of the European Union, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
D49805/02.1