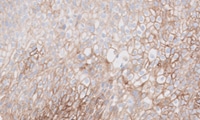
Head and Neck Squamous Cell Carcinoma (HNSCC): PD-L1 IHC 22C3 pharmDx

In HNSCC, PD-L1 testing with PD-L1 IHC 22C3 pharmDx can help identify patients for first-line treatment with KEYTRUDA1,2
- HNSCC is the seventh most common cancer worldwide3 and accounts for more than 90% of head and neck cancer cases.4 In the United States, approximately 65,000 new head and neck cancer cases are diagnosed annually5,6
- PD-L1 testing provides a direct assessment of PD-L1 expression, which is a biomarker for response to anti-PD-1 therapy in HNSCC1,2
- PD-L1 testing with PD-L1 IHC 22C3 pharmDx was used to assess PD-L1 expression in patients with metastatic or recurrent HNSCC in the KEYNOTE-048 clinical trial*1,2
* Review the PD-L1 IHC 22C3 pharmDx Instructions for Use for more information regarding the KEYNOTE clinical trials
The only PD-L1 assay used in the KEYTRUDA KEYNOTE-048 clinical trial in HNSCC1,2
PD-L1 expression in KEYNOTE-048 was determined by PD-L1 IHC 22C3 pharmDx, the FDA-approved companion diagnostic for KEYTRUDA1

† In the KEYTRUDA single agent and control arms
‡ Cetuximab, platinum, and FU
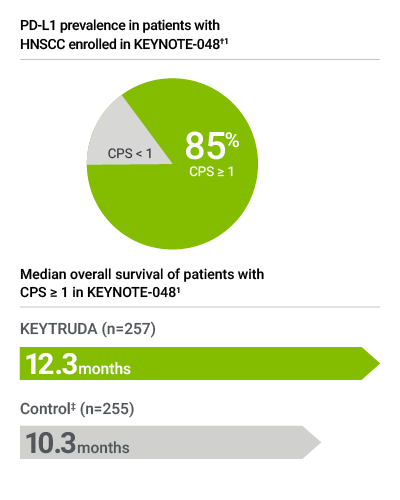
Note: PD-L1 expression level CPS ≥ 20 may be of interest to treating physician but does not determine eligibility for first-line treatment with KEYTRUDA as a single agent.
Forty-three percent of patients had tumors that expressed PD-L1 with CPS ≥ 20 (prevalence calculation based on patients with known PD-L1 expression [N=597]; 4 patients had unknown PD-L1 expression status). Median overall survival of patients in the CPS ≥ 20 subgroup was 14.9 months in the KEYTRUDA single agent arm, as compared to 10.7 months in the control arm.
When confidence in a PD-L1 test is critical, the ONE you choose is crucial
- The ONE PD-L1 assay used in KEYTRUDA clinical trials1,2
- The ONE PD-L1 assay first approved with KEYTRUDA in every indication that requires PD-L1 testing1,2
- The ONE PD-L1 assay trusted worldwide to test hundreds of thousands of patients for KEYTRUDA7


KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
References: 1. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2019. 2. Keytruda [package insert]. Kenilworth, NJ: Merck & Co., Inc.; 2019. 3. Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; Cheng, J.D.; Chow, L.Q. Safety And Clinical Activity of Pembrolizumab or Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-label, Multicenter, Phase 1b Trial. Lancet Oncol. 2016, 17 (7), 956-965. 4. Gupta, B.; Johnson, N.W.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology. 2016, 91, 13-23. 5. Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68 (1), 7-30. 6. Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69 (1), 7-34. 7. Data on file. Agilent Technologies, Inc.
For countries outside of the United States, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
D54671/01.1