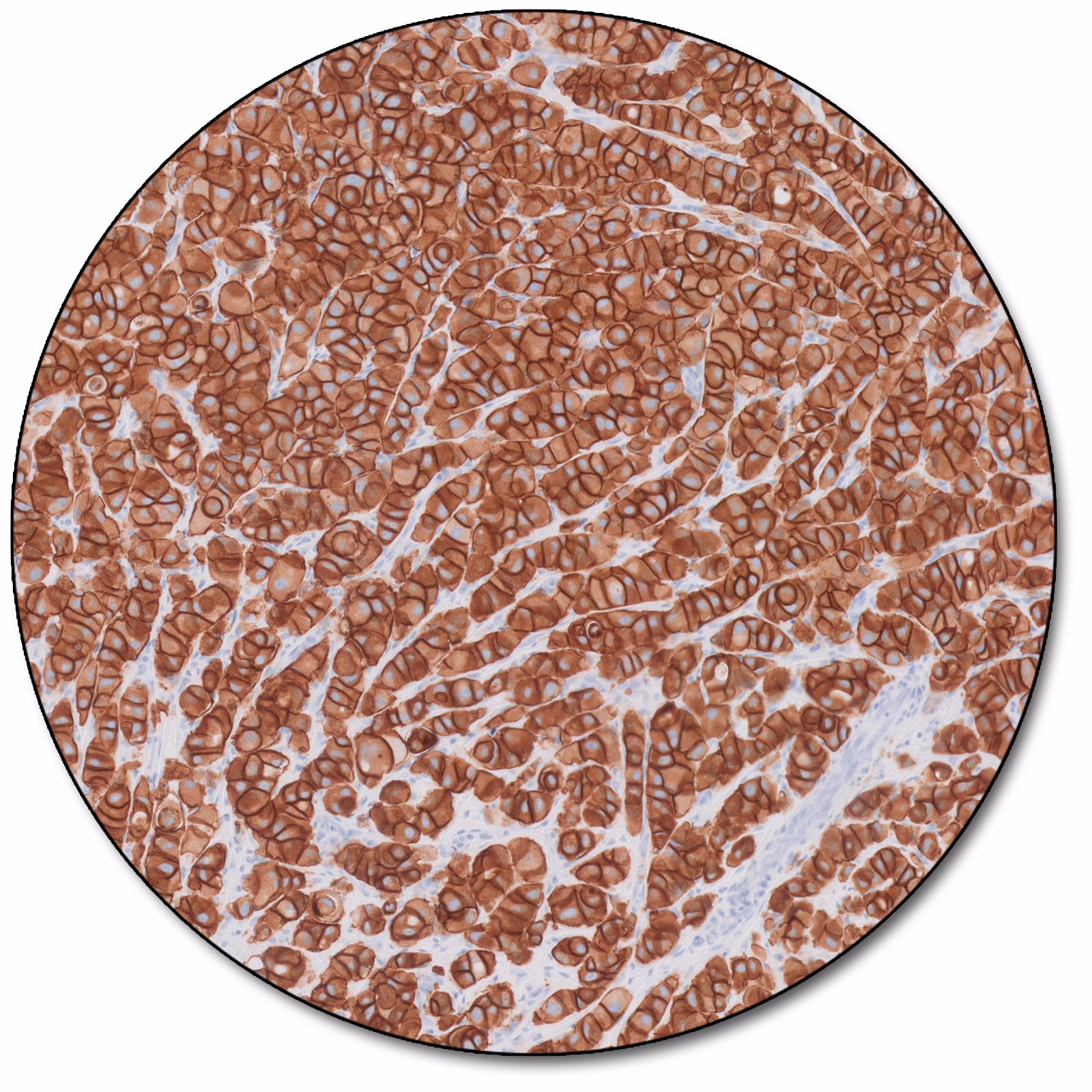
Breast carcinoma
HercepTest™ mAb pharmDx (Dako Omnis)

HercepTest™ mAb pharmDx (Dako Omnis) is a semiquantitative immunohistochemical assay based on a primary monoclonal rabbit antibody (clone DG44) and an assay-specific visualization reagent. The assay determines HER2 protein overexpression in formalin-fixed, paraffin-embedded (FFPE) breast cancer tissues processed for histological evaluation. HercepTest™ mAb pharmDx (Dako Omnis) is indicated as an aid in the assessment of breast cancer patients for whom Herceptin® (trastuzumab) treatment is being considered.
HercepTest™ mAb pharmDx (Dako Omnis) contains the reagents and protocol required to complete a fully automated immunohistochemical staining procedure of FFPE specimens using Dako Omnis.
HercepTest™ and Herceptin® are trademarks owned by Genentech, Inc. and/or F. Hoffmann-La Roche Ltd.; HercepTest™ is subject to an exclusive trademark license to Agilent Technologies, Inc.
The licensed antibody is created by Epitomics Inc. (an Abcam company), using Abcam’s proprietary rabbit monoclonal antibody technology covered under Patent Nos. 5,675,063 and 7,402,409.