Raman Spectroscopy
Innovative Raman Spectrometers Using SORS & TRS for Noninvasive Chemical Analysis
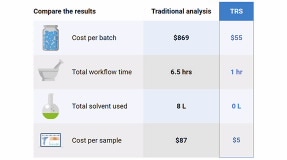
SORS enables our Raman spectrometers to analyze through containers and opaque barriers, from helping first responders identify the contents of suspect packages, to high-throughput raw material identification inside unopened packaging at pharmaceutical quality control. TRS nondestructively screens whole tablets and capsules in seconds, for rapid content uniformity and polymorph analysis in pharma QC and formulation development.