Gas Chromatography Fundamentals

What is Gas Chromatography?
Gas Chromatography – The Basics
Gas chromatography (GC) is a powerful analytical technique that can be used to separate, identify, and quantify individual chemical components in complex mixtures.
The word “gas” in GC does not refer to the type of samples the technique applies to, but rather the fact that a gas carries the sample through the instrument. This is called the carrier gas or mobile phase and is commonly high-purity helium, hydrogen, or nitrogen. Indeed, most GC methods target liquid samples and there are even applications for solids. The fundamental requirement for samples to be analyzed by gas chromatography is the compounds of interest in the sample must volatilize without thermally decomposing.
The Gas Chromatograph
The enormous breadth of GC system configurations and form factors can seem daunting, but every GC system can be dissected into three discrete parts: the inlet, the column, and the detector.
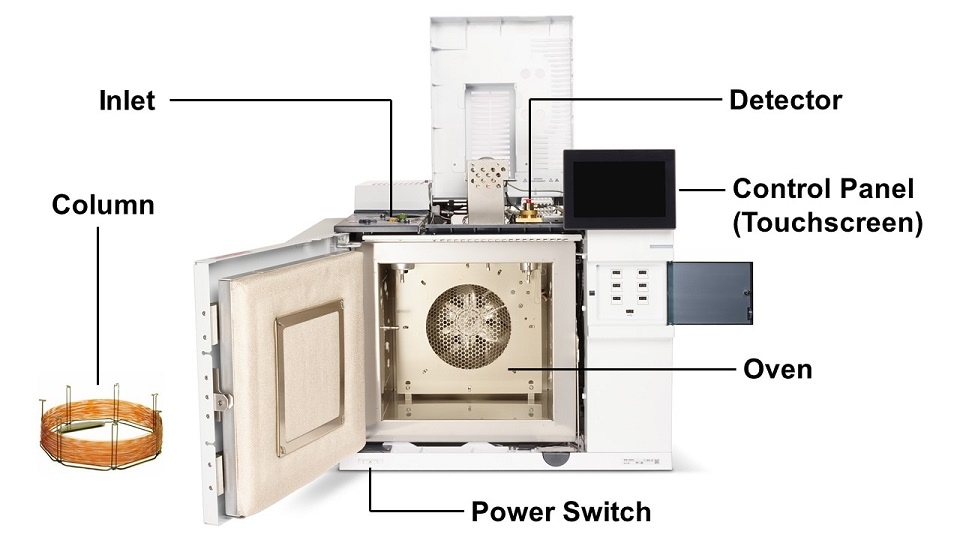
Each of these are temperature controlled (either held constant or ramped over the course of a sample analysis) using dedicated column ovens and heated zones, and each has one or several gases flowing through it at specific pressures or flow rates (also constant or ramped). The inlet facilitates the introduction of the sample into the gas chromatography system and ultimately onto the column. As the sample flows through the column, it separates into its individual components, which are then characterized by the detector. The most common GC configuration today is a split/splitless (SSL) inlet, a wall-coated open tubular (WCOT) capillary column containing a thin layer of dimethylpolysiloxane stationary phase, and a flame ionization detector (FID).
The GC Inlet
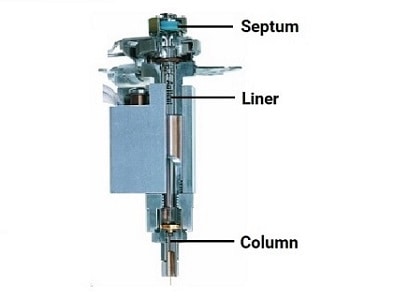
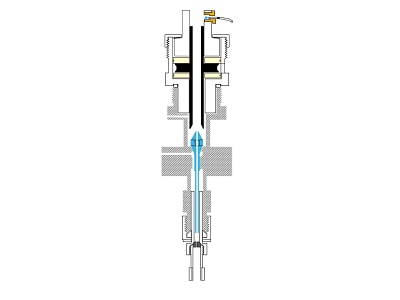
The inlet is the most complex part of many gas chromatography instruments. When analyzing liquids, samples are typically injected through a septum into the inlet using a syringe. An SSL inlet, as shown here, vaporizes the sample at temperatures up to 350 °C inside a glass liner. The liner provides an inert surface during vaporization to prevent the decomposition of active compounds in the sample that can be catalyzed by hot metal surfaces. The liner can also be packed with deactivated glass wool for more efficient vaporization and incorporate different internal geometries and baffles to increase mixing and homogenization of the sample vapor.
Once vaporized, the sample is swept onto the column by the carrier gas. Because the dimensions of the column are small and the sensitivity of the detector is high, very little of the vaporized sample needs to be swept onto the column. By employing a split gas flow, most of the sample vapor is discarded out the inlet vent, leaving only a small sharp plug of sample vapor at the head of the column. This discrete sharp sample plug typically yields the most efficient chromatographic performance of narrow peaks and high resolution, making split injection a popular configuration.
The SSL inlet can also operate in splitless mode where significantly more sample vapor flows onto the column. This mode yields increased sensitivity for trace-level analysis at the cost of increased peak broadening and thermal degradation of unstable analytes due to longer residence time of the sample in the inlet.
Other common gas chromatography inlets include:
- Programmable temperature vaporizer (PTV), which is similar to SSL but enables rapid ramping of the inlet temperature during injection.
- Cool on-column (COC), where the needle enters and injects the entire sample directly onto the head of the column at a lower temperature to further protect against possible thermal degradation and facilitate trace-level analysis.
- Multimode Inlet (MMI) that combines the flexibility of a standard split/splitless inlet with the capabilities of a PTV inlet.
The GC Column
Modern capillary GC columns are comprised of two main parts:
- A fused silica glass tube with an external polyimide coating.
- A stationary phase made of a thin film of a high molecular weight, thermally stable polymer coated onto the inner wall.
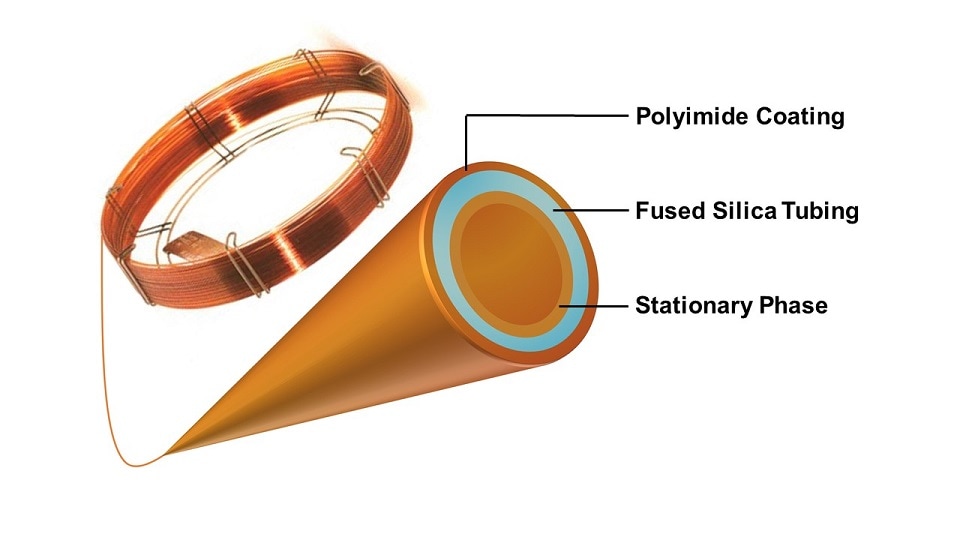
The fused silica tubing typically has an internal diameter of 0.1 – 0.53 millimeters and can reach lengths between 10 – 150 meters. The external polyimide coating dramatically increases the flexibility of the glass column and allows it to be tightly coiled and housed in the GC oven for temperature control.
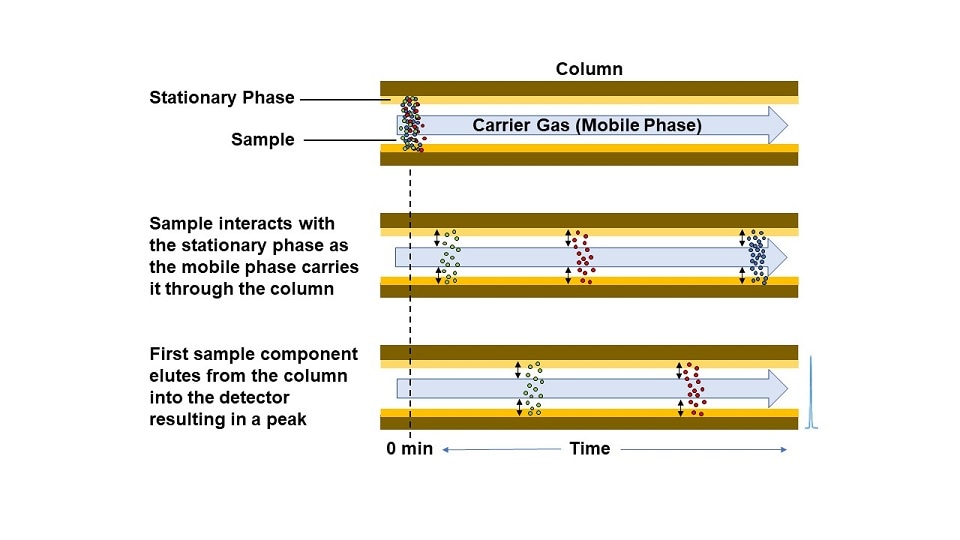
As the sample plug flows through the column, the different components separate from one another based on the strength of their interaction with the stationary phase. Many different types of stationary phases exist for gas chromatography and are usually described by their degree of polarity. While the intermolecular forces governing solubility equilibrium is a complex topic, a high-level description is polar molecules are more soluble in polar systems while non-polar molecules are more soluble in non-polar systems (“like dissolves like”). When a GC employs a polar stationary phase, the more polar molecules in a sample flowing through the column will partition more into the stationary phase compared to the non-polar molecules and will take longer to flow through the column. The degree of partitioning is also temperature-dependent, and the temperature of the oven that houses the column can be leveraged to facilitate improved separation between specific compounds with similar polarities. A linear temperature ramp of the column oven is common in many GC applications.
The GC Detector
As the separated components of the sample mixture arrive at the end of the column, the detector translates the amount of each component into an electrical signal. An FID uses a hydrogen flame to ionize the molecules as they elute from the column. These ions generate a change in current proportional to the number of molecules present. FIDs are the most common detectors used in hydrocarbon GC applications due to their high sensitivity, large linear dynamic range, and robust performance.
Another common gas chromatography detector is the thermal conductivity detector (TCD), which is not as sensitive as FID but is universally responsive to all compounds except the carrier gas. There are additional detector technologies that are very selective for specific types of organic compounds including:
- Electron capture detector (ECD) for halogenated and aromatic compounds, as well as other analytes with high electron affinity.
- Nitrogen-phosphorus detector (NPD) for compounds containing nitrogen or phosphorus.
- Flame photometric detector (FPD) for compounds containing sulfur or phosphorus.
- Chemiluminescence detectors for compounds containing sulfur (SCD) or nitrogen (NCD).
Mass spectrometry (MS) is a powerful analytical technique that can also be used as a detector for GC separations, and the combination of these techniques is called GC/MS. Like FID, MS systems produce an electrical signal proportional to the amount of each component present. However, the MS also provides information on the mass of the molecules detected, which makes GC/MS exceptionally effective at identifying unknown compounds in samples.

The Chromatogram
The data output of GC is a chromatogram which plots retention time (typically in minutes) on the x-axis and detector response (typically in picoamps) on the y-axis.
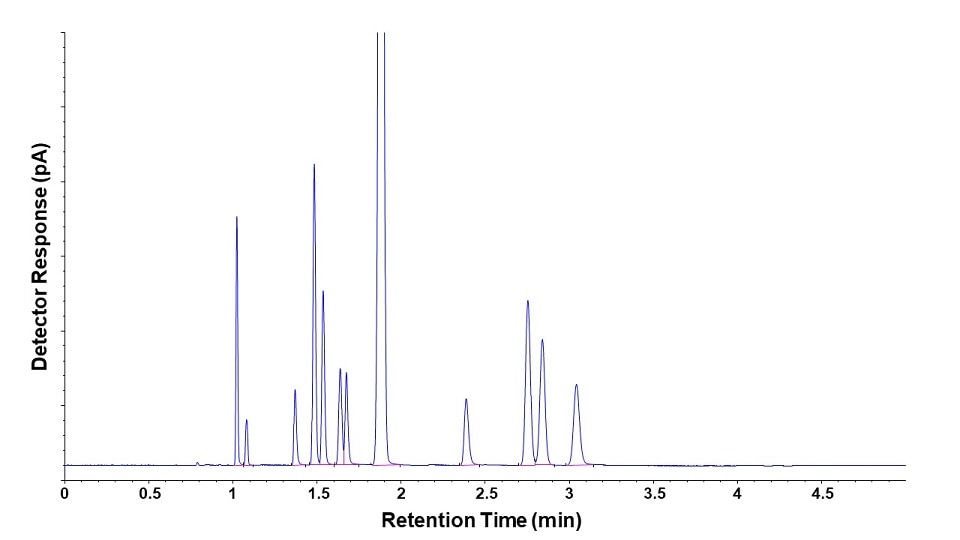
The retention time is a measure of how long it takes each compound band to travel from injection, through the column, and into the detector. This time is reproducible for a given set of instrument conditions (column, flows, pressures, temperatures, etc.).
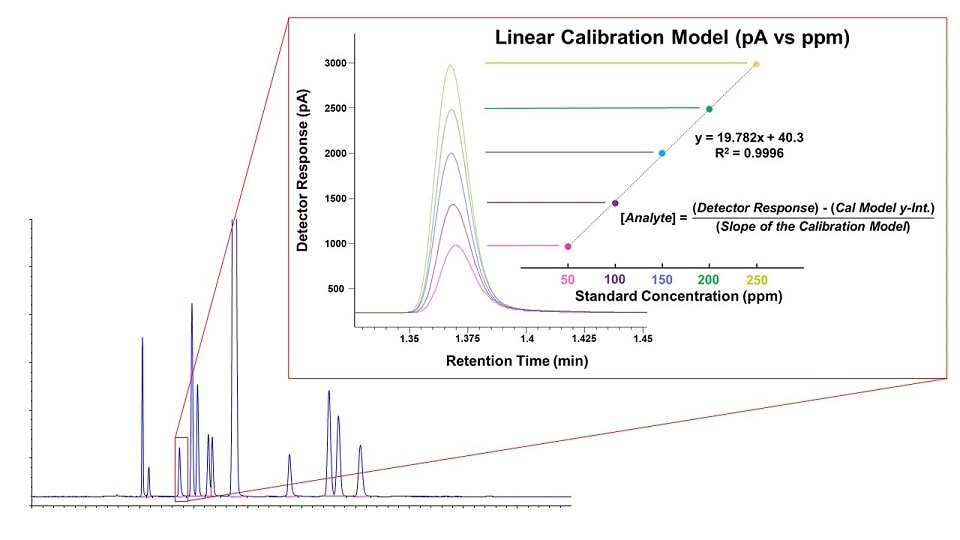
A common strategy in GC methodology involves running analytical standards containing known compounds of interest to determine their corresponding retention times. Comparing these to the retention times for peaks in the sample chromatogram can be used to identify unknown compounds. Running standard mixtures at several concentration levels and plotting against detector response results in a calibration curve that can then be used to determine the concentration of sample components. Calibration curves are fundamental to quantitative analysis in gas chromatography.
Additional information
Gas Chromatography Applications
Watch the four-part Fundamentals of GC video series on Agilent University (registration required).