Access Agilent eNewsletter, March 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Simplify workflows for dioxins and PCBs with new Agilent GC/MS/MS Dioxins in Feed and Food Analyzer
By Mike Vagner
Agilent GPSD Solutions Product Manager
Recently enacted European Union Commission regulations No 589/2014 and No 709/2014 have extended the confirmatory techniques for dioxin analysis to include the use of gas chromatography/tandem mass spectrometry (GC/MS/MS). Prior to these new regulations, labs were required to use high-resolution mass spectrometry to confirm and quantify dioxins. While the latter technique offers high sensitivity and selectivity, the hardware is very expensive and requires highly skilled operators. The new Agilent GC/MS/MS Dioxins in Feed and Food Analyzer provides a more routine, cost-effective way to accomplish high-quality dioxin analyses.
Dioxins are highly toxic compounds that are persistent organic pollutants (POPs). They originate as byproducts of industrial processes such as paper pulp bleaching and pesticide manufacturing. Other sources are combustion processes such as waste incineration. You may ingest dioxins through food of animal origin – such as meat, dairy products, and fish – which is why many food researchers and environmental agencies monitor these compounds. This newest addition to the Agilent range of dioxin solutions simplifies dioxin analyses.
 Enlarge
Enlarge
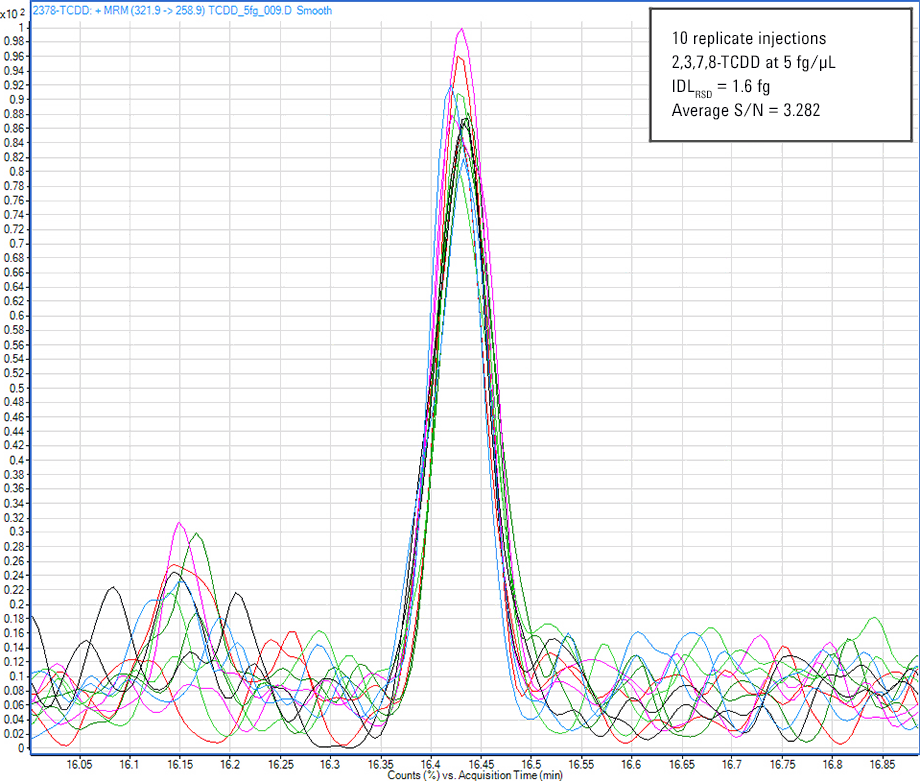
Figure 1. Analysis of 2,3,7,8-TCDD shows an instrument detection limit less than 5 fg for the Agilent 7010 Triple Quadrupole GC/MS. This sensitivity easily meets regulations.
Outstanding sensitivity – easily meet regulations
Agilent developed the new GC/MS/MS Dioxins in Feed and Food Analyzer so laboratories can perform confirmation and quantification of dioxins with GC/MS/MS, which is a more cost-effective and widely adopted technique than GC/high-resolution MS. Built upon the Agilent 7010 Triple Quadrupole GC/MS, which provides up to 10-fold more sensitive than competitive tandem quadrupole systems, this new analyzer provides the sensitivity required for trace dioxin and dioxin-like PCB detection.
For dioxin analysis in multiple reaction monitoring (MRM) mode, the instrument limit of quantitation (iLOQ) must be in the mid- to low-femtogram level to provide accurate quantitation at regulation levels. Figure 1 clearly demonstrates this capability.
Consistent, reliable results
The analyzer also uses the Agilent Multimode Inlet, which offers a wide range of injection techniques and injection volumes to optimize detection limits. The system includes Retention Time Locking (RTL) to PCB 105 at 15.00 minutes, in order to maintain consistent retention times and dependable data quality. The combination of these features delivers a more reproducible and rugged analysis.
Custom report saves time and complies with regulations
In addition, the Agilent GC/MS/MS Dioxins in Feed and Food Analyzer provides a more efficient workflow that greatly reduces the complexity of the analytical method and simplifies the complex calculations required for reporting. The dioxin analysis requires that you analyze two separate fractions from a single sample and combine their results. With this analyzer, both analyses use the same chromatographic conditions, which simplifies reporting of results.
 Enlarge
Enlarge
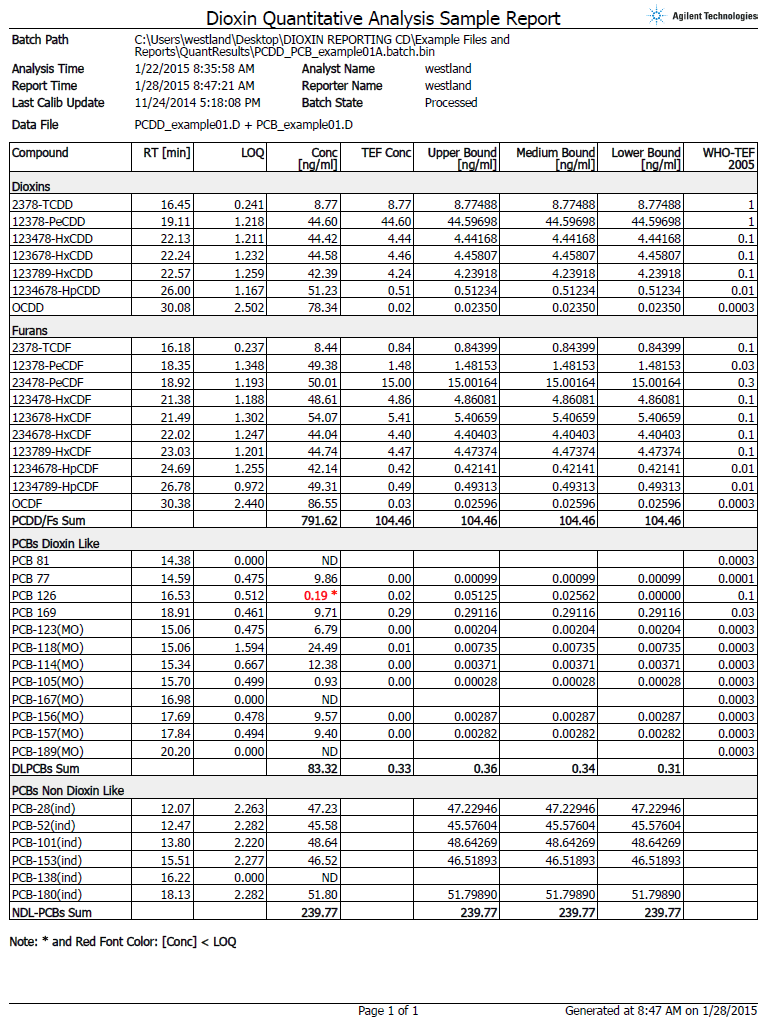
Figure 2. The automated report shown here eliminates the need for tedious and error-prone manual calculations.
European Union (EU) regulations mandate stringent reporting requirements. To achieve compliance, multiple complicated calculations and report metrics – such as limit of quantification (LOQ), toxic equivalency factor (TEF; which relates the toxicity of each individual analyte to 2,3,7,8-TCDD which is assigned a value of 1) – and the upper/lower boundary concentrations are required.
To meet these mandated requirements, the Agilent GC/MS/MS Dioxins in Feed and Food Analyzer includes a new custom supplemental software that automatically performs these calculations and generates a report that is compliant with the latest EU regulations (Figure 2).
Achieve regulatory compliance with consistent, efficient results using new dioxins analyzer
The Agilent GC/MS/MS Dioxins in Feed and Food Analyzer offers labs a cost-effective process to perform dioxin analyses. The analyzer’s custom reporting capabilities provide labs with an easy-to-use tool that saves time and demonstrates compliance with EU regulations.
To read more about dioxins, please visit the Agilent Dioxin Solutions homepage. Then, contact your Agilent Sales Representative today for additional information about the Agilent GC/MS/MS Dioxins in Feed and Food Analyzer.
>> Update My Profile | Subscribe to Access Agilent | Article Directory