Access Agilent eNewsletter, July 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Automated purification with seamless results transfer from analytical sub-2-micron UHPLC to preparative HPLC
By Andreas Tei
Agilent Product Manager for Preparative HPLC Systems
Chemists in drug discovery or agrochemical research labs synthesize a large number of compounds that require purification before biological assays can be started. Generic Ultra High Performance Liquid Chromatography (UHPLC) methods are ideal for high throughput labs to rapidly confirm the successful synthesis of the desired compounds in just a few minutes. However, the purification step that follows requires tedious scale up calculations from sub-2 µm analytical UHPLC columns to preparative columns to create optimized purification conditions. This process can be a challenge for more experienced chromatographers and almost unrealistic for less experienced users.
 Enlarge
Enlarge
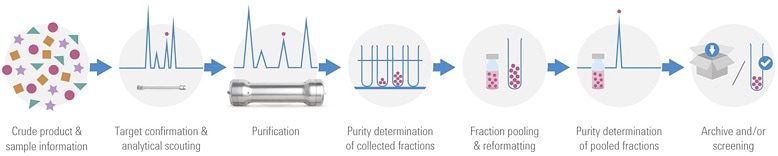
Figure 1. The purification workflow in medicinal chemistry.
Overcoming purification hurdles with Agilent
This challenge is the reason that research chemists are now turning to Agilent for simplified solutions for purification. By using Agilent’s OpenLAB CDS – Chemstation Edition, with the new Agilent Automated Purification Solution, high throughput labs can now overcome purification hurdles for a successful method transfer from analytical to preparative chromatography. The new software enables a successful automated high throughput compound purification process by applying tailored gradient profiles for each target compound. This feature has been combined with an automated tracking of samples and corresponding collected fractions during the entire purification workflow (shown in Figure 1).
Crude samples are often analyzed on UHPLC/Mass Spectometry systems.Target compounds are automatically identified and tailored gradient profiles for the purification step are generated to obtain an optimum resolution and process efficiency. After the purification step, the purity of the collected fractions is determined directly by spectral data information. Pure fractions of the same compound will be pooled, dried down, and resubmitted to UHPLC/MS for purity confirmation before archiving or submitting them to screening tests. The software tracks the submitted samples and the identity of the collected fractions through the entire purification process.
 Enlarge
Enlarge
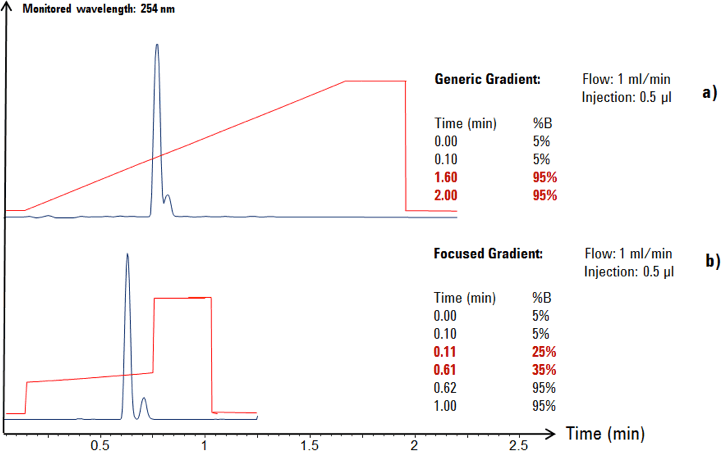
Figure 2. Generic gradient (a) compared to a focused gradient (b).
 Enlarge
Enlarge
Figure 3. Purification results after successful method transfer.
From analytical UHPLC to preparative chromatography
In this example, a generic gradient was applied for the analytical scouting run of the crude product on UHPLC columns. By using a generic gradient profile, the resolution was not yet optimized for this sample (see Figure 2a).
After a focused gradient profile (modified gradient profile) had been developed by a chromatographer, the target compound showed successful separation from its byproduct (Figure 2b). The focused gradient profile was transferred to the larger preparative column dimensions using established mathematical equations [1,2].
Baseline separated compounds were obtained from the preparative system after a successful method transfer from sub-2 µm columns to a 5 µm particle column (Figure 3).
To learn more about this method transfer process from UHPLC to preparative High Performance Liquid Chromatography (HPLC), please see Agilent technical publication 5991-2013EN.
Challenges in purification processes
In high throughput labs where the purification of more than 50 different samples per day/system is required, it is nearly impossible to write tailored gradients for each individual sample. The same holds true for labs where the resources of chromatographers are limited. Until now, the workaround has involved selecting from a suite of pre-defined preparative solvent gradient profiles. As a result, maximum column loadability, purity, and recovery for the purification step have often been compromised. Automated tracking of injected samples and their corresponding fractions is required to minimize process errors.
 Enlarge
Enlarge
Figure 4. Automated Purification Software, analytical results browser.
No more compromised purification steps
Now, with new Agilent automated purification software for OpenLAB – Chemstation Edition, these steps are no longer compromised. After uploading an analytical sample result set which has been acquired on an Agilent UHPLC/MS system to the newly designed Automated Purification Software, tailored gradient profiles for each sample will be generated and applied during the purification step.
Tailored gradient profiles and spectral data can be reviewed by the use of the analytical result browser (Figure 4). The gradient profiles and identified targets can also be modified by the operator. After the purification step has been completed, all relevant information from the collected fractions can be reviewed by the use of the fractions result browser. A tracking file from the injected sample to the collected fraction is created in *.CSV format for further use.
A fully-integrated and scalable workflow-focused solution
The Agilent Automated Purification Software works on the Agilent 1260 Infinity Preparative-Scale LC/MS Purification System, a scalable, workflow-focused solution for medicinal drug discovery and agrochemical research. Built on robust 1260 Infinity UHPLC and preparative HPLC modules, as well as proprietary software algorithms, the system provides superior purification with automated delay volume calibration and focused gradients tailored on the fly to individual target compounds.
There are many benefits to using the Agilent 1260 Infinity Preparative Scale LC/MS Purification System in your lab. These include:
- True Automated Purification – no manual method development for purification processes.
- Automated method transfer from analytical to prep columns independent from the desired flow rates, column dimensions and particle sizes.
- Use of optimized (focused) preparative gradient profiles for each sample generated by a mathematical algorithm on the fly to obtain the best resolution and process efficiency.
- Support of automated purification workflows which includes the import and export of sample sequences, analytical result sets, the purification step, and the re-analysis and re-formatting of collected fractions.
- EasyPrep mode with a simplified user interface for a streamlined workflow for walk up users.
Agilent: one stop for all of your UHPLC, HPLC, and MS needs
You can learn more about Agilent Automated Purification Software in the OpenLab CDS Data Sheet and the Automated Workflow Solution for Preparative Chromatography technical overview. Additionally, you can find more information on the OpenLAB – Chemstation Edition by visiting our site. Agilent also provides a full range of UHPLC, HPLC, and MS solutions, explore these applications today.
References:
- D. Guillarme, D. Nguyen, S. Rudaz, J.L. Veuthey, Eur. J. Pharm. Biopharm., 2007, 66, 475-482.
- D. Guillarme, D. Nguyen, S. Rudaz, J.L. Veuthey, Eur. J. Pharm. Biopharm., 2008, 68, 430-440
>> Update My Profile | Subscribe to Access Agilent | Article Directory