Access Agilent eNewsletter, August 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Software assisted genotoxic impurity identification by Q-TOF LC/MS
By Siji Joseph, Syed Salman Lateef, Vinayak AK, and Sreelakshmy Menon
Agilent Application Scientists
Purity of a drug substance is critical to ensure drug safety and efficacy, but oxidative degradation of drugs during handling or storage may lead to the formation of impurities that are genotoxic. Given the emphasis of regulatory authorities on rigorous control of genotoxic impurities in drug products, labs are challenged with the difficult tasks of monitoring and identifying these impurities at trace levels. In a recent study, we successfully used an Agilent software-assisted quadrupole time-of-flight (Q-TOF) LC/MS method to screen and identify potential trace-level genotoxic degradants from atorvastatin – a drug that is commonly marketed as Lipitor.
 Enlarge
Enlarge
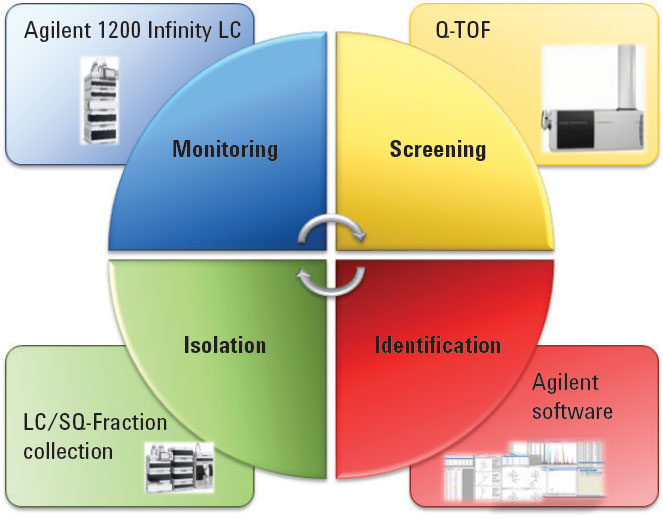
Figure 1. This multistep workflow was successfully accomplished with a versatile suite of Agilent hardware and software products.
 Enlarge
Enlarge
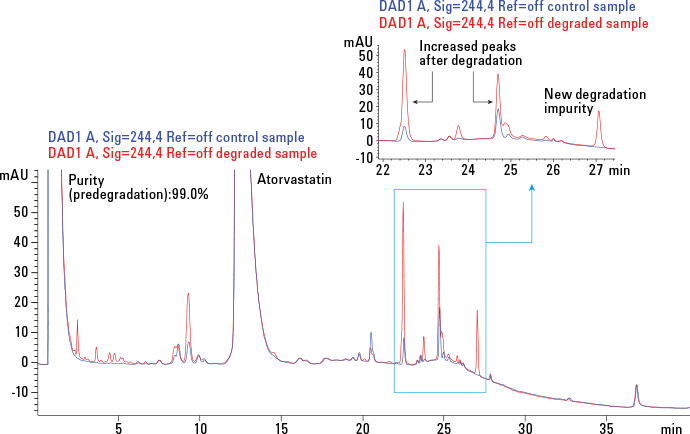
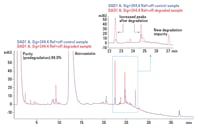
Figure 2. Monitoring the extent of degradation using an HPLC-UV method. UV traces at 244 nm of nondegraded (blue) and degraded (red) samples are overlaid.
 Enlarge
Enlarge
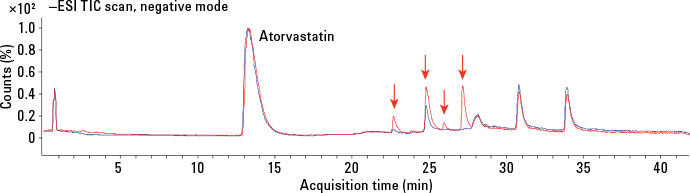
Figure 3. Overlaid TICs for nondegraded sample (blue) and degraded sample (red). Arrows point to significant peaks in degraded sample.
Figure 1 shows the workflow we used for the study. We accomplished the oxidative degradation of atorvastatin calcium drug substance by treating the compound with 3 percent hydrogen peroxide at 40 °C for 24 hours.
HPLC method shows the extent of degradation
We monitored the extent of degradation with an Agilent 1290 Infinity LC System using a USP liquid chromatography method (Figure 2). In the figure, a portion of the LC chromatogram is expanded to demonstrate the formation of both new impurities and impurities that increased in abundance. We operated the LC system with Agilent OpenLAB CDS ChemStation Edition (version C.01.05).
The individual modules of the Agilent 1290 Infinity LC System used in this example included:
- Agilent 1290 Infinity Binary Pump
- Agilent 1290 Infinity Autosampler with an Agilent 1290 Infinity Thermostat
- Agilent 1290 Infinity Thermostatted Column Compartment
- Agilent 1290 Infinity Diode Array Detector with 60-mm Max-Light flow cell
Q-TOF LC/MS analysis screens for impurities
To screen for impurities, we analyzed samples with and without degradation using an Agilent 6530 Accurate Mass Quadrupole Time-of-Flight (Q-TOF) LC/MS System with an Agilent Jet Steam (AJS) dual source. The HPLC/Q-TOF analyses in both positive and negative ion modes confirmed the presence of degradants. Figure 3 shows overlaid total ion chromatograms (TICs) of nondegraded and degraded samples. We used Agilent MassHunter Workstation software (version B.05.01) to acquire the data.
Powerful software tools help find and identify genotoxic impurities
Differential impurities between control (nondegraded) and degraded samples were extracted by Agilent MassHunter Profinder software (version B.06.00) and subsequently analyzed with Agilent Mass Profiler Professional (MPP) statistical analysis software (version 12.65). Statistical analysis of the results from degraded and nondegraded samples showed that approximately 15 compounds had two-fold changes. This list includes up-regulated and newly formed impurities found in the degraded sample.
We used accurate mass data of those 15 compounds to search with ID Browser or the Find by Formula algorithm in the Agilent MassHunter Qualitative Analysis software. We searched an accurate mass database that we developed with Agilent MassHunter PCDL Manager (version B.04.00). The database included accurate masses and formulas of known impurities and degradants of atorvastatin. This search resulted in identification of five known impurities.
The precursor and fragment ion information of unknown impurities processed by the Agilent MassHunter Molecular Feature Extraction (MFE) algorithm was imported to Agilent Molecular Structure Correlator (MSC) software to propose possible structures. Details are discussed in Agilent Application Note 5991-4404EN. Consistent with available literature, we found that three of the 15 impurities contain genotoxic epoxide and hydroperoxide functional groups. We compared the MS/MS fragmentation pattern of these genotoxic impurities with known similar structures to confirm the findings.
Fraction collection enables impurity isolation
Fractions of the genotoxic impurities were isolated from the complex degradation sample using the Agilent 1290 Infinity LC System coupled with the Agilent 6150 Single Quadrupole LC/MS and the Agilent 1260 Infinity Bio-inert Fraction Collector. You can scale up this fractionation process to get sufficient amounts of the impurity for further structure confirmation with techniques like NMR.
From this study we demonstrated the successful use of a variety of Agilent hardware and software solutions for the structural identification of potential genotoxic impurities that originate from forced degradation of a drug substance. This study also proves that you can easily monitor thermally labile genotoxic impurities like epoxides with the Agilent Jet Stream source.
Learn how you can achieve results like these
You can find additional details and more results in Agilent Application Note 5991-4404EN. Then talk with your Agilent Representative to ensure that you have everything you need to identify drug impurities.
>> Update My Profile | Subscribe to Access Agilent | Article Directory