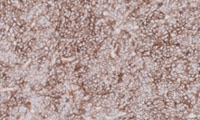
PD-L1 IHC 22C3 pharmDx for Triple-Negative Breast Cancer

In TNBC, PD-L1 testing with PD-L1 IHC 22C3 pharmDx can help identify patients for treatment with KEYTRUDA1,2
- In the United States, breast cancer is the most common type of cancer diagnosed in women,3 and approximately 15–20% of breast cancer diagnoses are TNBC.4 Patients with TNBC have significantly worse overall survival times than non-TNBC patients5
- PD-L1 testing provides a direct assessment of PD-L1 expression, which is a biomarker for response to anti-PD-1 therapy in TNBC1,2
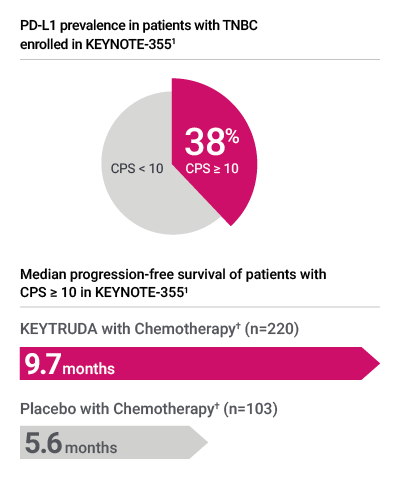
- PD-L1 testing with PD-L1 IHC 22C3 pharmDx was used to assess PD-L1 expression in patients with locally recurrent unresectable or metastatic TNBC in the KEYNOTE-355 clinical trial*1,2
* Review the PD-L1 IHC 22C3 pharmDx Instructions for Use for more
information regarding the KEYNOTE clinical trials
PD-L1 IHC 22C3 pharmDx was the only PD-L1 assay used in the KEYTRUDA KEYNOTE-355 clinical
trial in TNBC1,2
PD-L1 expression in KEYNOTE-355 was determined by PD-L1 IHC 22C3 pharmDx, the FDA-approved companion diagnostic for KEYTRUDA1

† Chemotherapy: paclitaxel, paclitaxel protein-bound, or gemcitabine and carboplatin
When confidence in a PD-L1 test is critical, the ONE you choose is crucial
- The ONE PD-L1 assay used in KEYTRUDA clinical trials1,2
- The ONE PD-L1 assay first approved with KEYTRUDA in every indication that requires PD-L1 testing1,2
- The ONE PD-L1 assay trusted worldwide to test hundreds of thousands of patients for KEYTRUDA6


Intended Use
For in vitro diagnostic use.
PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using monoclonal mouse anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) triple-negative breast cancer (TNBC) tissue using EnVision FLEX visualization system on Autostainer Link 48.
Triple-Negative Breast Cancer (TNBC)
PD-L1 protein expression in TNBC is determined by using Combined Positive Score(CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100. The specimen should be considered to have PD-L1 expression if CPS ≥ 10.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying TNBC patients for treatment with KEYTRUDA® (pembrolizumab).
For descriptions of the intended use in other indications, please refer to the current version of the Instructions for Use (IFU) for PD-L1 IHC 22C3 pharmDx, Code SK006.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
References: 1. PD-L1 IHC 22C3 pharmDx [Instructions for Use]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2020. 2. Keytruda [package insert]. Kenilworth, NJ: Merck & Co., Inc.; 2020. 3. Cancer of the Breast (Female) ‑ Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/breast.html (accessed Sep 15, 2020). 4. Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T. J.; Deng, X. Triple‑negative breast cancer: is there a treatment on the horizon? Oncotarget 2017, 8 (1), 1913–1924. 5. Li, X.; Yang, J.; Peng, L.; Sahin, A. A.; Huo, L.; Ward, K. C.; O’Regan, R.; Torres, M. A.; Meisel, J. L. Triple-Negative Breast Cancer Has Worse Overall Survival and Cause-Specific Survival than Non-Triple-Negative Breast Cancer. Breast Cancer Res Treat. 2016, 161 (2), 279–287. 6. Data on file. Agilent Technologies, Inc.
For countries outside of the United States, see the local KEYTRUDA product
label for approved indications and expression cutoff values to guide therapy.
D62315/01.1