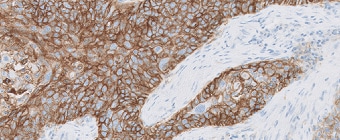
PD-L1 IHC 22C3 pharmDx for Esophageal Squamous Cell Carcinoma

In ESCC, PD-L1 testing with PD-L1 IHC 22C3 pharmDx can help identify patients for treatment with KEYTRUDA1,2
- In the United States, esophageal cancer is expected to cause approximately 16,000 deaths in 2019.3 ESCC accounts for approximately 30% of all esophageal cancers diagnosed in the United States4 and has a 5-year survival rate of 12%5
- PD-L1 testing provides a direct assessment of PD-L1 expression, which is a biomarker for response to anti-PD-1 therapy in ESCC1,2
- PD-L1 testing with PD-L1 IHC 22C3 pharmDx was used to assess PD-L1 expression in second-line patients with metastatic or locally advanced, unresectable ESCC in the KEYNOTE-181 clinical trial1,2
The only PD-L1 assay used in the KEYTRUDA KEYNOTE-181 clinical trial1,2
PD-L1 expression in KEYNOTE-181 was determined by PD-L1 IHC 22C3 pharmDx, the FDA-approved companion diagnostic for KEYTRUDA1

*Prevalence calculation based on patients with known PD-L1 expression (patients with unknown PD-L1 expression, n=6) and excludes patients with specimens outside the stability window (n=28)
†Calculation excludes 10 patients with specimens outside the stability window
‡KEYNOTE-181 evaluated overall survival in second-line patients with metastatic or locally advanced, unresectable ESCC
§Either paclitaxel, docetaxel, or irinotecan

When confidence in a PD-L1 test is critical, the ONE you choose is crucial
- The ONE PD-L1 assay used in KEYTRUDA clinical trials1,2
- The ONE PD-L1 assay first approved with KEYTRUDA in every indication that requires PD-L1 testing1,2
- The ONE PD-L1 assay trusted worldwide to test hundreds of thousands of patients for KEYTRUDA6


KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
References: 1. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2019. 2. Keytruda [package insert]. Kenilworth, NJ: Merck & Co., Inc.; 2019. 3. American Cancer Society. Facts & Figures 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf (accessed July 31, 2019). 4. SEER Cancer Statistics Review 1975-2016. National Cancer Institute. https://seer.cancer.gov/csr/1975_2016/ (accessed July 31, 2019). 5. Kahn, A.; Crowell, M.D.; Fleischer, D.E. Reducing the Risk of Esophageal Squamous Cell Carcinoma: Out with the Old; in with the New. Gastrointestinal Endoscopy. 2019, 89(4), 733–735. 6. Data on file. Agilent Technologies, Inc.
For countries outside of the United States, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
D54362/01.1