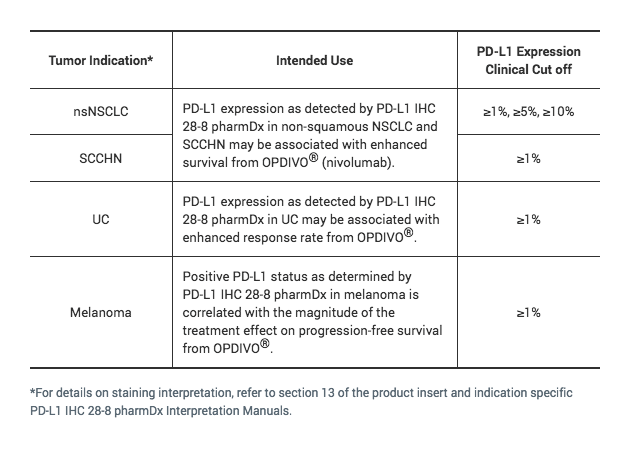
PD-L1 IHC 28-8 pharmDx for SCCHN


PD-L1 IHC 28-8 pharmDx for SCCHN
The only FDA approved test for PD-L1 expression that may be associated with enhanced survival from OPDIVO® (nivolumab) for SCCHN1, 2
Demonstrated clinical results
PD-L1 IHC 28-8 pharmDx is the first fully validated and clinically relevant test for OPDIVO in SCCHN.
Detection of PD-L1 expressing tumor cells in SCCHN patient specimens may indicate an enhanced survival benefit to OPDIVO (nivolumab) treatment for the patient.2
The CHECKMATE-141 study demonstrated a statistically significant improvement in overall survival (OS) for subjects randomized to nivolumab as compared to investigator’s choice at a pre‑specified interim analysis (78% of the planned number of events for final analysis). The median OS was 7.5 months for nivolumab subjects compared to 5.1 months for investigator’s choice subjects with a hazard ratio (HR) of 0.70 (95% CI 0.53, 0.92).
Summary of OS by PD-L1 IHC 28-8 pharmDx expression level and treatment group1
Data from a pre-specified exploratory analysis (N=260) of CHECKMATE-141 (N=361).
| Tumor PD-L1 Expression | <1% | ≥1% | ||
|---|---|---|---|---|
| Nivolumab | Investigator’s Choice | Nivolumab | Investigator’s Choice | |
| Median OS | 5.7 mos. | 5.8 mos. | 8.7 mos. | 4.6 mos. |
| Hazard Ratios | 0.89 (95% CI: 0.54, 1.45) | 0.55 (95% CI: 0.36, 0.83) | ||
| Abbreviations: CI = confidence interval | ||||
361 patients were randomized at 55 sites in 15 countries to one of two treatment arms (240 nivolumab vs. 121 to investigator’s choice) and stratified according to prior cetuximab treatment (yes/no). The major efficacy outcome measure for CHECKMATE-141 was Overall Survival (OS). Additional efficacy outcome measures included Progression Free Survival (PFS) and Objective Response Rate (ORR).
Frequency of PD-L1 Expression in Quantifiable* Samples from SCCHN—CHECKMATE-1411
| PD-L1 Expression | Nivolumab (N=161) | Investigator’s Choice (N=99) | Total (N=260) |
|---|---|---|---|
|
≥1% PD-L1 Expression Subjects |
88 (54.7%) | 61 (61.6%) | 149 (57.3%) |
|
<1% PD-L1 Expression Subjects |
73 (45.3%) | 38 (38.4%) | 111 (42.7%) |
| *260 of 327 samples were PD-L1 quantifiable from study CHECKMATE-141. | |||
Baseline SCCHN Specimen Origin—Study CHECKMATE-1411
Tumor specimens were collected from SCCHN tumors from either a primary or metastatic site, consistent with the inclusion requirements for the study. 327 subjects (out of 361 total subjects) had tumor tissue collected at baseline with the following site proportion:
|
Primary Tumor 29.7%(97/327) |
Metastatic Tumor 52.0%(170/327) |
Not reported 18.3%(60/327) |
Robust performance
PD-L1 IHC 28-8 pharmDx is fully validated for analytical performance, having met stringent acceptance criteria for ultimate quality results.
|
Selected analytical validation parameters |
Results for SCCHN |
|---|---|
| Analytical specificity |
|
| Sensitivity |
|
| Repeatability |
|
| External reproducibility |
|
| ANA = Average Negative Agreement | APA = Average Positive Agreement | OA = Overall Agreement | |
Order information
| Product | Code |
|---|---|
| PD-L1 IHC 28-8 pharmDx | SK005 |
| Required but not included in kit: | |
| Autostainer Link 48 | AS480 |
| EnVision FLEX Wash Buffer, 20x | K8007 |
| EnVision FLEX Hematoxylin (Link) | K8008 |
| PT Link | PT101/PT200 |
| PT Link rinse station | PT109 |
References
- Clinical Trial: CHECKMATE-141, CA209141
- PD-L1 IHC 28-8 pharmDx Instructions for Use