Access Agilent eNewsletter October 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Innovative mass spec design speeds drug quantitation in heavy matrices
By Craig P. Love, Behrooz Zekavat, and Patty Sun
Agilent Mass Spec Division
Pharmaceutical drug discovery requires many in vitro and in vivo assays to screen potential drug candidates for further development. In vivo identification and quantification of metabolites are specifically challenging due to the presence of matrix components. These investigations require the use of sensitive, robust analytical techniques for high-throughput screening of drug candidates. The most common analytical approach is to use triple quadrupole mass spectrometers in multiple reaction-monitoring (MRM) mode. However, the analytical performance of these systems can degrade over time as nonvolatile sample matrix components accumulate in the ion source and transfer optics. As a result, frequent cleaning may be required to maintain optimal performance.
Robust analytical performance
In examining the challenges of drug quantitation, we evaluated the analytical robustness of the Agilent 6470 Triple Quadrupole LC/MS, coupled to an Agilent RapidFire 365 High-throughput MS System. For drug discovery organizations, Agilent RapidFire 365 delivers automated, high-capacity sample analysis, which is a more successful approach to high throughput screening of challenging drug targets. RapidFire 365 also provides 10x faster throughput for MS-based analyses, such as in vitro ADME assays.
The Agilent 6470 Triple Quadrupole LC/MS solution features several key design innovations for sensitive, precise, and robust quantification of target analytes in heavy sample matrices over a wide linear dynamic range. These innovations include:
- Optimized ion optics and prefilter geometries to increase ion transmission and minimize contamination
- A curved and tapered hexapole collision cell that enables high-efficiency MS/MS fragmentation and transmission of ions
- An ion detector with a high-energy conversion dynode and low noise characteristics that enables efficient positive and negative ion detection across a wide m/z range
 Enlarge
Enlarge
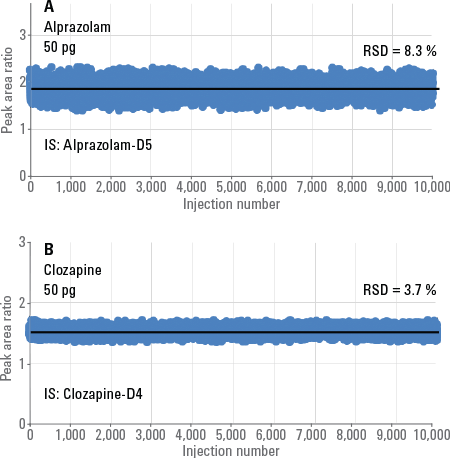
Figure 1. Peak area ratios for alprazolam (A) and clozapine (B) over 10,000 injections.
 Enlarge
Enlarge
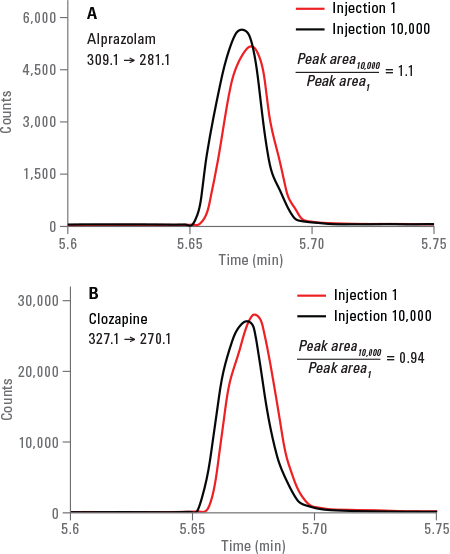
Figure 2. Overlaid MRM chromatograms for alprazolam (A) and clozapine (B) at the beginning and end of 10,000 injections.
For this investigation, using the Agilent 6470 Triple Quadrupole LC/MS and Agilent RapidFire 365 HTMS combo, we tested a mixture of alprazolam and clozapine, and their corresponding internal standards (alprazolam-D5 and clozapine-D4, respectively) spiked in precipitated porcine plasma at 5 ng/mL (each). Pig plasma was precipitated using a 1:3 volume ratio of acetonitrile followed by centrifugation at 13,500 rpm for five minutes. The supernatant was then diluted with water (1:10) before spiking with the analytes and internal standards. The spiked plasma sample was transferred to 96-well microtiter plates and sealed using an Agilent PlateLoc Thermal Microplate Sealer before RapidFire/MS analysis.
The Agilent RapidFire 365 HTMS parameters were optimized to yield the RapidFire wash solvent conditions. Wash solvent 1 (buffer A at 1.50 mL/min) was water plus 2 mM ammonium acetate and 0.1 % formic acid. Wash solvent 2 (buffer B at 1.25 mL/min) comprised 20% acetonitrile in water plus 10 mM ammonium acetate and 0.1 formic acid. An elution solvent (buffer C, 75% ethyl acetate in methanol) at 1.25 mL/min was used to elute analytes from RapidFire Type A (C4) cartridges.
This system configuration analyzed small molecule pharmaceuticals in plasma at about 15 seconds per sample, over five consecutive days (2,000 injections/day). Figure 1 shows the peak area ratios for alprazolam and clozapine from the injection of 50 pg of each analyte as a function of RapidFire injection number (a total of 10,000 injections). Stable peak area ratios were obtained over the 10,000 injections, demonstrating the sound analytical performance of the Agilent 6470 Triple Quadrupole LC/MS.
The overlaid MRM chromatograms for the drugs at the beginning and end of the 10,000 sample injections are shown in Figure 2. Peak shape and area (height) for alprazolam and clozapine remain unchanged after 10,000 injections.
It’s clearly evident that the innovative ion transfer optics design of the Agilent 6470 Triple Quadrupole LC/MS minimizes the adverse effects of sample accumulation, providing a robust analytical platform for the ultra-high-throughput analysis of target drug candidates in precipitated plasma samples.
Further details of this investigation are freely available in Agilent publication 5991-5953EN.
Agilent offers a broad array of separation and detection solutions
Long-standing Agilent expertise in measurement drives the development of tomorrow’s streamlined engines of discovery. With innovations in separation and detection technologies, including the new Agilent 1290 Infinity II LC, analytical HPLC and UHPLC columns, the Agilent 6500 Series Accurate-Mass Quadrupole Time-of-Flight LC/MS, and the Agilent 6495 Triple Quadrupole LC/MS, you can count on superior sensitivity and data quality for profiling, identifying, characterizing, and quantifying compounds of interest.
Our comprehensive solutions are optimized to provide the data you need to propel the drug discovery process forward, giving you full confidence every time you make a go, no-go decision. Explore the entire family and see what Agilent pharma solutions can do for you.
>> Update My Profile | Subscribe to Access Agilent | Article Directory