Access Agilent eNewsletter, January 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Superior visibility and more confident dissolution testing with new surveillance solution
By Dan Spisak
Agilent Product Manager, Dissolution Systems
Observation is a critical component of dissolution testing. Visibility into the dissolution vessel permits the analyst to assess the initial disintegration or patterns of the dosage form during the dissolution process. It also ensures proper sampling position, which could affect results for certain products. As with most parts of the dissolution technique, consistency is a key factor for producing repeatable results.
A drawback of the visual confirmation process is that dissolution methods for extended-release products may last for many hours. Even for immediate-release products that require only 30-minute tests, constant observation for the duration of the experiment becomes a time-consuming task. Another issue would be for light sensitive products, where tinted glass or a shielded waterbath is used to protect the dosage form from photo-degradation, restricting the view into the vessel itself.

Figure 1. Visual observation of dissolution testing reveals sources of variability.
As stated in the US Pharmacopeia General Chapter <1092>, which details the Dissolution Procedure, “visual observations are often helpful for understanding the source of the variability and whether the dissolution test itself is contributing to the variability” (Figure 1). Adherence to this recommendation is simplified by automating the monitoring, recording and subsequent documentation of critical dissolution test events that might otherwise have gone unnoticed. This provides a useful benefit in formulation development, potential failure investigations or even as a supplement to analytical results.
Without an analyst’s presence, strategically placed cameras and corresponding software can greatly improve monitoring of dissolution test conditions and/or potential failure analysis. Specifically:
- Positive dosage form introduction
- Positioning of the dosage form (center, off-center)
- Hydrodynamics within the dissolution vessel
- Particle behavior
- Proper de-aeration of dissolution media
- Vessel visibility of light sensitive products
- Sampling timing and position
- Aberrant data investigation
- Documentation of uncharacteristic behavior
For light sensitive products – where photo-degradation of the active drug is a concern – cameras may deliver a solution for better visibility that prevents the need for special laboratory lighting or other measures that would inhibit traditional observation of the dissolution vessels.
Adding this capability to your lab’s dissolution environment offers important insight at each stage of the pharmaceutical product development process. Whether it’s during formulation optimization, method development, or further downstream during stability or routine QA/QC testing, observation and recording of the dissolution test itself provides an ideal mechanism for additional information gathering. A system such as this could be utilized to evaluate how unexpected results are obtained, or periodically, to assess dosage form performance. Either way, failure investigations are expedited with an increasing amount of evidence available and the dissolution data is enhanced with supplemental images or videos.
Agilent and Merel partner to deliver integrated dissolution surveillance system
 Enlarge
Enlarge
Figure 2. dissoGUARD® Surveillance System paired with Agilent Dissolution Apparatus.
 Enlarge
Enlarge
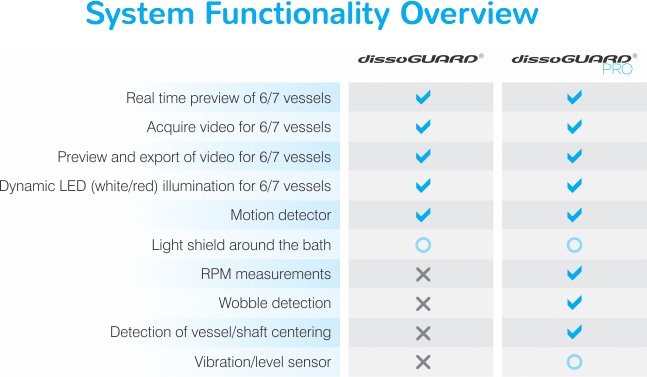
Figure 3. dissoGuard® provides data on key physical parameters.
Agilent has partnered with Merel d.o.o. to provide a dissolution surveillance system – dissoGuard® – capable of capturing vital information that was previously unattainable. With cameras placed beneath each individual vessel, no additional bench space is required (Figure 2). The dissoGuard® software gives the user a real-time view as well as preset recording options. The videos or snapshots can then be marked with key events such as dosage introduction, sampling or unusual behavior, and then easily exported for sharing or documentation purposes.
In addition to the cameras beneath the vessels, an external camera is employed for an alternate view – this location is determined at your discretion. The data collected by each camera does not simply record events, but can also provide information regarding key physical parameters such as RPM, vessel-to-shaft centering and wobble (see Figure 3). The dissoGuard® Pro software can alert the user of any abnormal status of these parameters during a test, preventing lost time and aiding in eventual failure investigations. An innovative lighting system using white/red LEDs that are manually or automatically adjusted to red lighting allow for observation of light-sensitive products as well.
Designed to support both the Agilent 708-DS and 709-DS Dissolution Apparatus, the dissoGuard® Surveillance System adds superior visibility to the dissolution test environment.
Agilent offers a vast array of dissolution solutions
Agilent offers a portfolio of dissolution products that meet all USP, EP, and JP guidelines and integrate the latest technological advances backed by comprehensive service, support, and training. You can view these dissolution instruments in action by viewing the Agilent Dissolution Demo at Your Desk.
Additionally, you can check out the comprehensive Dissolution 1-on-1 Training Course on Agilent’s interactive resource site – the Agilent Dissolution Exchange. The exchange provides a wealth of information on dissolution, support for solving laboratory challenges, and an opportunity to discuss dissolution topics with other analysts. To learn more about the dissoGuard® Surveillance System contact your Agilent Sales Representative today.
>> Update My Profile | Subscribe to Access Agilent | Article Directory