Access Agilent eNewsletter February 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Rapid, non-invasive oral fluid testing solution from Agilent and Immunalysis
By Christine Moore
VP R&D, Immunalysis Corporation
and Tom Gluodenis
Agilent World Wide Forensics Industry Manager
Oral fluid analysis is increasingly seen as a reliable method for detecting drugs. Workplace drug testing, school programs, and the criminal justice system have embraced oral fluid testing which has several distinct advantages over blood and urine testing. The technique uses simple, rapid, non-invasive, and observable specimen collection that can provide evidence of being “Under the Influence." Drugs in oral fluid show some correlation with plasma drug levels and have a detection window of one to two days, i.e. demonstrating recent use. In addition, adulteration is difficult and the parent drug is usually detected. Finally, improved oral fluid technology expands opportunities in drug test profiles.
Agilent has partnered with the Immunalysis Corporation to develop the first end-to-end workflows for detection of over 100 different drugs from 11 drug classes in oral fluids. All of the workflows are now available in a free Agilent application compendium, Drugs of Metabolites in Oral Fluid: Immunoassay Screening and LC/MS/MS Confirmation and Quantification. This 100-page compendium includes methods for many routine drugs of abuse as well as synthetic cannabinoids and schedule II prescription medications. In this article, we highlight just one of these protocols.
Quick, easy detection of synthetic cannabinoids in oral fluid
Spice is a generic name for several synthetic cannabinoids. Many are banned by the U. S. Drug Enforcement Agency and other administrations around the world. These compounds can cause tachycardia (racing heartbeat), seizures, hypertension, chest pain, palpitations, and hallucinations. Toxic symptoms usually last no longer than two to four hours with usually no long-lasting residual adverse effects. However, long-term effects are unknown, though it is likely that JWH-018, for example, can precipitate psychosis in vulnerable individuals.
We developed and validated a procedure using the Quantisal™ oral fluid collection device [1]. The collection device consists of a tube containing transport buffer and an absorbent pad that the donor places in his or her mouth to collect saliva.
Sample preparation was achieved with an Agilent Bond Elut Plexa solid phase extraction cartridge. An Agilent ZORBAX Extend-C18, 2.1 x 50 mm, 1.8-µm LC column was employed for separation. Because LC/MS for oral fluid analysis is increasingly favored and often mandated, we used an Agilent 6430 Triple Quadrupole LC/MS System.
For this protocol, we began by validating the experimental design with seven synthetic cannabinoids (Table 1).
|
Synthetic cannabinoid |
||||||
JWH-018 |
JWH-073 |
JWH-200 |
JWH-250 |
CP 47497 |
CP 47497 C8 |
HU-210 |
|
LOQ (ng/mL) |
0.5 |
0.5 |
0.5 |
2 |
0.5 |
2 |
5 |
|
Intra-day imprecision (%) |
||||||
4 ng/mL |
3.9 |
3.6 |
5.0 |
3.4 |
4.9 |
3.9 |
8.6 |
40 ng/mL |
2.2 |
2.1 |
6.0 |
2.0 |
4.1 |
4.3 |
5.6 |
|
Inter-day imprecision (%) |
||||||
4 ng/mL |
8.8 |
9.6 |
6.2 |
11 |
7.7 |
11 |
10 |
40 ng/mL |
8.5 |
7.9 |
6.2 |
11 |
10 |
11 |
12 |
|
Pad recovery (%) |
||||||
4 ng/mL |
65.5 |
67.4 |
85.0 |
66.5 |
77.7 |
76.0 |
86.4 |
40 ng/mL |
70.6 |
61.4 |
81.4 |
75.1 |
71.3 |
78.2 |
75.7 |
Matrix effect (%) |
-55 |
-45 |
-55 |
-73 |
-64 |
-55 |
-49 |
Process efficiency (%) |
40 |
51 |
56 |
24 |
38 |
45 |
51 |
Table 1. Validation results for synthetic cannabinoids in oral fluid using the Immunalysis Corp. collection pad with Agilent sample prep and LC/MS/MS.
Next, using the same experimental setup, we assessed recoveries of synthetic and naturally occurring cannabinoids, as shown below in Table 2.
 Enlarge
Enlarge
Figure 1. Synthetic cannabinoids in the saliva of two different subjects; BP = Blueberry Posh and BM = Black Mamba.
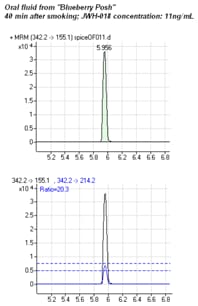 Enlarge
Enlarge
Figure 2. MRM chromatograms of oral fluid from a spice smoker sampled 40 minutes after smoking. The lower trace shows the LOQ with ± 20% ion ratio limits.
Drug |
Pad recovery (%) |
|
|---|---|---|
4 ng/mL |
40 ng/mL |
|
JWH-018 |
65.5 |
70.6 |
JWH-073 |
67.4 |
61.4 |
JXH-200 |
85.0 |
81.4 |
JWH-250 |
66.5 |
75.1 |
CP 47497 |
77.7 |
71.3 |
CP 47497 C8 |
76.9 |
78.2 |
HU-210 |
86.4 |
75.7 |
THC |
89.4 |
|
9-Carboxy-THC |
|
80.1 (10 ng/mL) |
2-Carboxy-THC |
78.2 |
|
Cannabinol |
79.7 |
|
Cannabidiol |
71.9 |
|
Table 2. Percentage recoveries of synthetic and naturally occurring cannabinoids at different concentrations using the Immunalysis Corp. collection pad.
Spice samples purchased legally, with the brand names Blueberry Posh and Black Mamba, were then tested. The drugs were smoked by two different subjects, whose saliva was sampled before and after smoking at intervals up to 12 hours. The samples were stored for one year at 4 °C for further analysis.
Following a single occasion of smoking, JWH-018 was still detectable 12 hours later in the saliva of the Blueberry Posh smoker. Concentrations were virtually identical in samples from this smoker after refrigerated storage in buffer for one year. However, concentrations had degraded in samples from the Black Mamba smoker (much lower levels), as shown above in Figure 1. Figure 2 shows MRM chromatograms of saliva from the Blueberry Posh smoker.
A full range of Agilent toxicology solutions
Agilent’s publication, Drugs of Metabolites in Oral Fluid: Immunoassay Screening and LC/MS/MS Confirmation and Quantification is a valuable asset to help your method development. Download your free copy today. This compendium is just one the many technical resources Agilent offers for drug testing labs. Explore the full range of options for oral fluid analysis and toxicology techniques, to see what Agilent can do for your lab.
References
>> Update My Profile | Subscribe to Access Agilent | Article Directory

