Access Agilent eNewsletter, November 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Pathway discovery using metabolomic profiling in prostate cancer research
By Arun Sreekumar
Baylor College of Medicine, Houston, TX
and Nigel Skinner
Agilent Global Segment Marketing Manager, Disease Research & Toxicology
Historically, the study of cancer has been dominated by its genetic underpinning. More recently, altered metabolism has received renewed attention as a key hallmark of cancer- and metabolism-focused research. Cancer metabolomics research is used to find diagnostic biomarkers, as well as to obtain more insight into cancer and carcinogenesis. Metabolite levels integrate the effects of gene regulation, post-transcriptional regulation, pathway interaction, and environmental perturbation. This downstream synthesis of diverse signals ultimately makes metabolites direct molecular indicators of cell status, which reflect a meaningful physiological phenotype.
Metabolomics provides a comprehensive profiling of metabolites in a sample, whether intracellular or from circulating biofluids. The ability of metabolomics to measure high-throughput, system-wide phenotypes gives it great power in oncology research.
Metabolomic profiling identifies biochemical pathways
Despite recent developments, castration-resistant prostate cancer (CRPC) is still the second-leading cause of cancer-associated mortality in American men, and its biological underpinnings are not well understood. Metabolomic profiling was recently used to identify biochemical pathways associated with CRPC [1], as well as potential biomarkers of bladder cancer progression [2]. The team measured levels of 150 metabolites and examined the rate of use of 184 metabolites in metastatic androgen-dependent (AD) prostate cancer and CRPC cell lines using a combination of targeted mass spectrometry (MS) and metabolic phenotyping. Metabolic data derived biochemical pathways that were enriched in CRPC, using Oncomine Concept Maps (OCM). The enriched pathways were then examined in silico for their association with treatment failure (that is, prostate-specific antigen (PSA) recurrence or biochemical recurrence) using published, clinically-annotated gene-expression data sets. Nineteen metabolites were altered in CRPC compared to AD cell lines. These altered metabolites mapped to a highly interconnected network of biochemical pathways that described UDP glucuronosyltransferase (UGT) activity.
The authors discovered an association with time-to-treatment failure when using genes restricted to this pathway in three independent gene-expression data sets. This highlights the value of employing metabolomic strategies in cell lines to derive potentially useful predictive tools for clinical research.
 Enlarge
Enlarge
Figure 1. Workflow used by Kaushik et al. in the metabolomic profiling of castration-resistant prostate cancer [1].
An Agilent 1260 Infinity Binary LC was used for the analysis. The measurement of metabolite levels was based on multiple-reaction monitoring (MRM), using 12 different methods with reversed-phase (RP) or aqueous normal-phase separation, prior to MS with an Agilent 6430 Triple Quadrupole LC/MS. An Agilent ZORBAX Eclipse XDB-C18 column was used for RP separation. Figure 1 is an outline of the experimental approach used to define the metabolic signature associated with CRPC.
High-throughput methods in cancer research
Cancer is a complex disease governed by multiple alterations of transcriptomics, proteomics, and metabolomics. Recent developments in high-throughput technologies have strengthened the methods used to integrate reproducible data from different platforms to determine a global picture of the pathogenesis of cancer.
One such study in breast cancer integrated high-throughput gene expression and proteomics data with biological network information, to determine defining molecular components of breast tumorigenesis [3]. This and similar studies highlight the power of an integrated pathway-centric approach to studying cancer. Kaushik et al. employed a similar approach but with a distinct method to combine (steady-state levels) data from metabolomics with those from gene expression in a pathway-centric manner, further validating the (flux) activity of identified pathways using metabolic phenotyping microarrays. Their approach identified biochemical pathways that are associated with the development of CRPC, as well predicting primary treatment failure or biochemical recurrence.
The potential of metabolomics
Metabolomics holds great promise for advancing the understanding, diagnosis, and treatment of cancer. The approach has already been used to uncover and verify mechanisms of carcinogenesis and proliferation, identify numerous candidate diagnostic biomarkers in biofluid and biopsy samples, and has even contributed to the staging of cancers and characterization of treatment efficacy. Much of this was done before metabolomics analysis became more widely accessible to researchers via the broader establishment of metabolomic labs. Cancer research metabolomics offers great promise, though with significant challenges. An obvious goal is to translate metabolomic measurements into a deeper biological understanding of the condition, ultimately enabling better drug design and development. An increasingly popular approach is through the integration of multiple “omics”. For example, integration of transcriptomic and metabolomic data has enabled deeper analysis of chemosensitive pathways and breast cancer, which may provide further validation and understanding, and thus potential clinical applications.
Agilent integrated solutions for integrated biology
Agilent has analytical products across the four major omics to help you take advantage of integrated multi-omics discovery and move forward with integrated biology. Take a look at the latest version of our GeneSpring GX bioinformatics software designed for pathway-centric multi-omic data integration. Also, be sure to look at Agilent workflows look at Agilent workflows ehensive overview of discovery metabolomics and check out our primer on the use of MS in metabolomics.
References:
- A.K. Kaushik, et al., J. Proteome Res., 2014, 13, 1088-1100.
- N. Putluri, et al., Cancer Res., 2011, 71, 7376-7386.
- M. Imielinski, et al., Mol. Cell. Proteomics, 2012, 11, M111.014910.
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Figure 1.
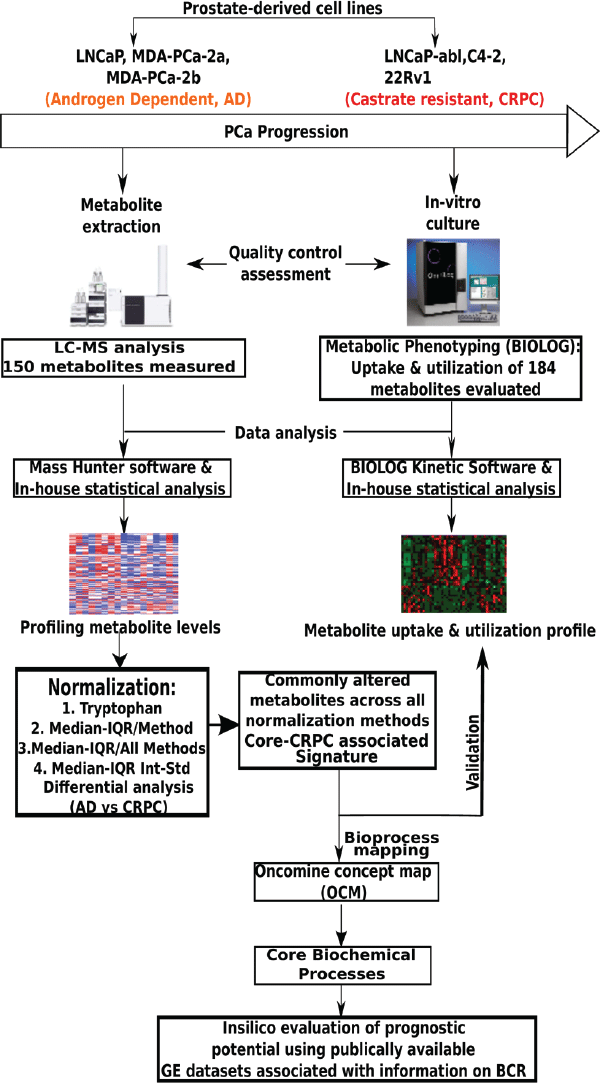
Workflow used by Kaushik et al. in the metabolomic profiling of castration-resistant prostate cancer [1].