Access Agilent eNewsletter, May 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Optimizing biosimilar titers with Agilent Bio-Monolith Protein A
By Phu Duong, James Martosell, and Maureen Joseph
Agilent Biocolumn Applications
During a recent study, Agilent Bio-Monolith Protein A columns were used to illustrate the separation and monitoring of a trastuzumab-biosimilar titer for cell-clone selection. Protein A purification of the trastuzumab biosimilar was followed by mass spectral (MS) analysis to demonstrate the power of the Agilent Bio-Monolith Protein A column for cell culture selection and optimization in determining trastuzumab glycosylation profiles.
Bio-Monolith Protein A is one of a family of Agilent Bio-Monolith HPLC columns for the analysis of macro bio-molecules.
 Enlarge
Enlarge
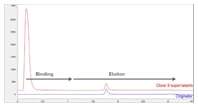
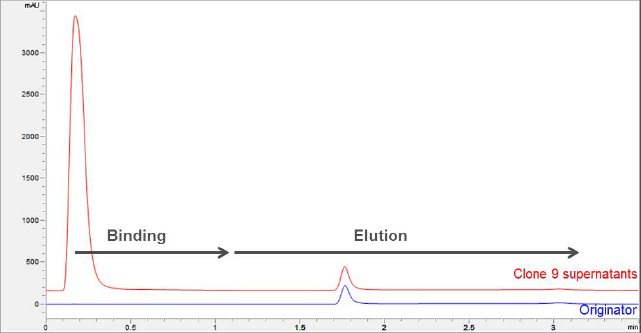
Figure 1. Titer analysis is used to compare a trastuzumab biosimilar and unbound proteins from harvested cell lysis with the trastuzumab originator.
Easy profiling of a trastuzumab glycosylation
Figure 1 shows the titer analysis of the sample lysis containing trastuzumab biosimilar and unbound proteins from harvested cell lysis, with a direct comparison to the trastuzumab originator. An overlay is shown between the 0.5 mg/mL trastuzumab originator and selected clone (#9) of 0.5 mg/mL trastuzumab biosimilar, using elution with 0.1 M citric acid, pH 2.8. The mAb peak area and retention positions comparisons between the trastuzumab originator and biosimilar are well matched.
After protein A purification of the trastuzumab biosimilar (clone #9), mAb peaks were collected and all were reduced to light and heavy chains. Several parameters, including molecular weight of the light and heavy chains and glycosylation, were examined by LC/MS following desalting. Although the molecular weight of the biosimilar clones was a good match to the originators, a closer look showed there were some differences in the glycosylation pattern. G0F was over-expressed in the clones and G1F was under-expressed. Therefore, the clone titers of the biosimilars needed tuning to match the characterization profile (glycosylation) of the originator.
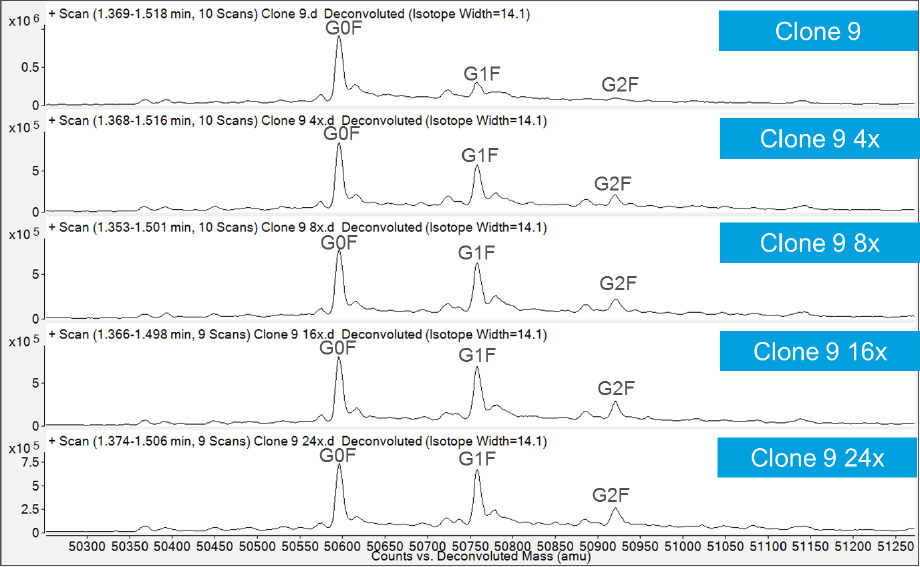
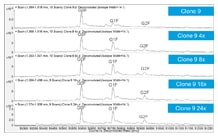
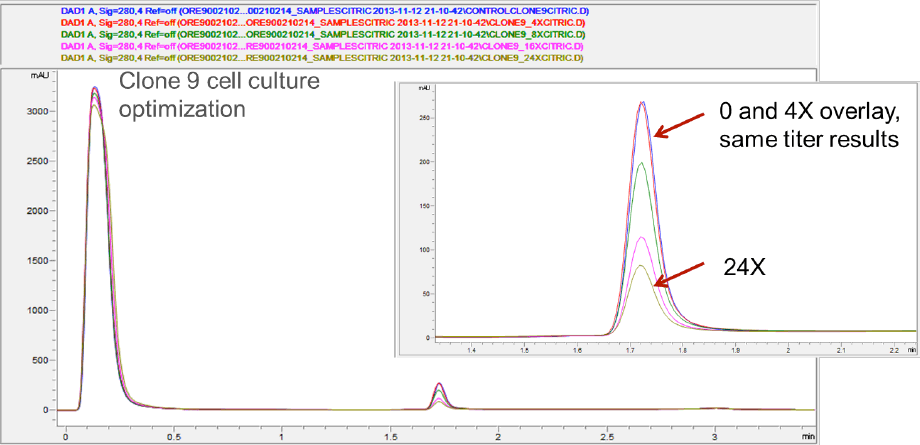
Compared to the originators, the clone-derived biosimilars contained less galactose, while G0F predominated. To bring the glycosylation within the originator specifications, cell culture medium was tuned by adding uridine, galactose, and manganese at different concentrations (4x, 8x, 16x, 24x). The Agilent Bio-Monolith Protein A column was used to determine the titers after tuning. Figure 2 shows example titer results from the clone 9 cell-culture optimization. No additional nutrients and the 4x level provided comparable results. You can see that 24x gave substantially lower levels.
 Enlarge
Enlarge
Figure 2. Example titer results from the clone 9 cell-culture optimization, with comparable results from no additional nutrients and 4x concentration, whereas 24x delivers substantially lower levels.
 Enlarge
Enlarge
Figure 3. Glycosylation tuning results of clone #9 trastuzumab biosimilar.
 Enlarge
Enlarge
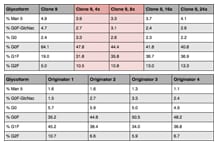
Table 1. Evidence that the trastuzumab-cloned biosimilar matches the originator drug.
For this analysis, we used LC/MS to assess adjustment of G0F and G1F levels. G1F increased dramatically, starting at the 4x level (Figure 3). Table 1 compares four batches of the originator to the biosimilar #9 clones. It is clear from the results that G0F was reduced and G1F increased, such that we were now in line with the originator drug. Evidence confirms that we have tuned the cell culture to drive the production of the biosimilar to match the original.
Achieve identical primary sequences
The originator and clone-derived trastuzumab (after tuning) displayed the same light chain and heavy chain molecular weights. US and European regulatory authorities consider this to be the most important attribute of biosimilarity, i.e. the primary sequence should be identical. The heavy chain of the originators and clone-derived mAb both contained the same glycans, but while the glycosylation was similar from a qualitative perspective, there were quantitative differences.
The method outlined here brings the glycosylation of clone-derived trastuzumab within the originator specifications. The titer decreased with increasing concentrations of manganese, uridine, and galactose. The Agilent Bio-Monolith Protein A column enabled workflow clone selection and purification, and provided critical titer measurements while tuning the trastuzumab biosimilar to the originator.
High resolution and rapid separations with Agilent Bio-Monolith HPLC columns
Agilent Bio-Monolith HPLC columns provide high resolution and rapid separations of IgG and IgM antibodies, plasmid DNA, viruses, phages, and other macro bio-molecules. The product family offers strong cation-exchange, strong and weak anion-exchange, as well as Protein A phases. All of the Bio-Monolith HPLC columns are compatible with HPLC and preparative LC systems.
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Figure 1.
Titer analysis is used to compare a trastuzumab biosimilar and unbound proteins from harvested cell lysis with the trastuzumab originator.
Figure 2.
Example titer results from the clone 9 cell-culture optimization, with comparable results from no additional nutrients and 4x concentration, whereas 24x delivers substantially lower levels.
Table 1
Glycoform |
Clone 9 |
Clone 9, 4x |
Clone 9, 8x |
Clone 9, 16x |
Clone 9, 24x |
|---|---|---|---|---|---|
% Man 5 |
4.9 |
3.9 |
3.3 |
3.7 |
4.1 |
% G0F-GlcNac |
4.7 |
2.7 |
3.1 |
2.4 |
2.6 |
% G0 |
2.4 |
3.3 |
2.6 |
2.3 |
2.2 |
% G0F |
64.1 |
47.8 |
44.4 |
41.8 |
40.8 |
% G1F |
19.0 |
31.8 |
35.8 |
36.7 |
36.9 |
% G2F |
5.0 |
10.5 |
10.8 |
13.0 |
13.3 |
Glycoform |
Originator 1 |
Originator 2 |
Originator 3 |
Originator 4 |
|---|---|---|---|---|
% Man 5 |
1.6 |
1.6 |
1.3 |
1.1 |
% G0F-GlcNac |
1.5 |
2.7 |
3.3 |
2.4 |
% G0 |
5.7 |
5.9 |
5.0 |
4.9 |
% G0F |
35.2 |
44.8 |
50.5 |
48.2 |
% G1F |
45.2 |
38.4 |
34.0 |
36.8 |
% G2F |
10.7 |
6.6 |
5.9 |
6.7 |
Evidence that the trastuzumab-cloned biosimilar matches the originator drug.