Access Agilent eNewsletter, February 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Comprehensive forensic/toxicology drug screening
By Trisa Robarge
Agilent Product Manager – Sample Prep
Screening for the presence of drugs in biological samples for forensic/toxicology analyses can present challenges because analyses must be both rapid and extremely reliable. You must avoid false positives, meet stringent chain-of-custody protocols, maximize uptime, and keep end-to-end workflows running smoothly, from sample preparation to final report. Agilent provides the tools that help you meet these challenges and to confidently perform routine analyses related to regulated/nonregulated urine, blood, and oral fluids; driving under the influence/forensic drugs; post-mortem toxicology; and doping control.
We highlight here two LC/MS methods for screening drugs in forensic/toxicology applications, using either simple dilute-and-shoot injection of urine or solid phase extraction for a more complex biological matrix such as plasma. Full details of the methods presented are found in Agilent application notes 5991-1667EN and SI-01014.
Dilute-and-shoot analysis of urine – in only minutes
In the first example, we developed a method to quantitate 65 drugs and metabolites in human urine using the Agilent 1260 Infinity Binary LC and Agilent 6420 Triple Quadrupole LC/MS. We prepared calibrators by spiking drug-free human urine with 1,000 ng/mL of all 65 compounds. After dilution and enzymatic hydrolysis, followed by further dilution, we injected samples onto the LC/MS/MS system. An Agilent Poroshell 120 SB-C18 column provided the separation. The development of the analytical methods, which ranged from custom to comprehensive, was quick and easy with the Agilent Forensic/Toxicology Database. The Agilent dynamic multiple reaction monitoring (dMRM) approach ensured accurate and reproducible quantitation with this type of analysis.
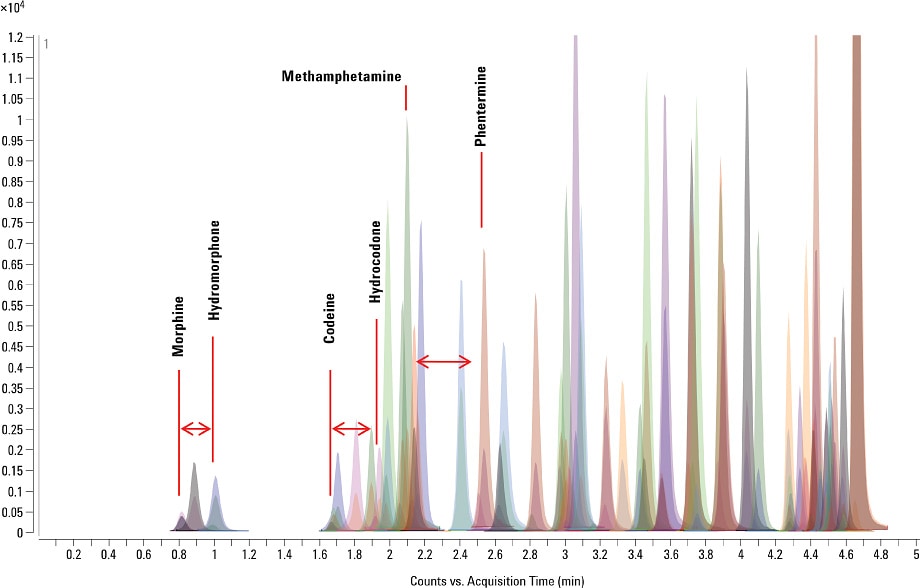
The Agilent system allowed the LC/MS/MS instrument to analyze a greater number of analytes in a given time frame without compromise of data integrity. In doing so, we were able to develop a highly comprehensive method that was capable of faster throughput than that achieved with traditional MRM. With this method, we analyzed a wide selection of analyte components and their isotopically labelled internal standards in only six minutes. It was important to obtain good baseline separation for the isobaric analytes in this comprehensive suite. We achieved this separation for morphine/hydromorphone, codeine/hydrocodone, and methamphetamine/phentermine.
 Enlarge
Enlarge
Figure 1. Maximize throughput: chromatography of 65 drugs and metabolites separated in a single run of just six minutes.
We met all typical detection limits under these extreme acquisition conditions, with all the analytes exhibiting better than 98 percent accuracy for each respective linear range. We measured detection limits conservatively by using signal-to-noise determinations along with percent coefficient of variation (%CV) precision data for the less abundant qualifier ions associated with each analyte. Figure 1 shows an overlaid chromatogram of a matrix-spiked sample of all analytes, together with the corresponding isotopically labeled internal standards. [1]
Sample preparation using dilute-and-shoot is often appropriate for drugs in urine. However, solid phase extraction may offer a more reliable and consistent approach for extraction of drugs from urine as well as more biologically complex samples such as plasma, whole blood, or tissue.
Solid phase extraction of drugs in plasma, with excellent recoveries
Biological samples can be complicated to analyze because proteins, peptides, salts, phospholipids, and other endogenous compounds are present in the matrix. Sample cleanup is therefore necessary to remove these inferences without significant loss of the target analytes. Solid phase extraction using simplified methods for routine analysis represents an ideal choice for removal of matrix and concentration of target analytes.
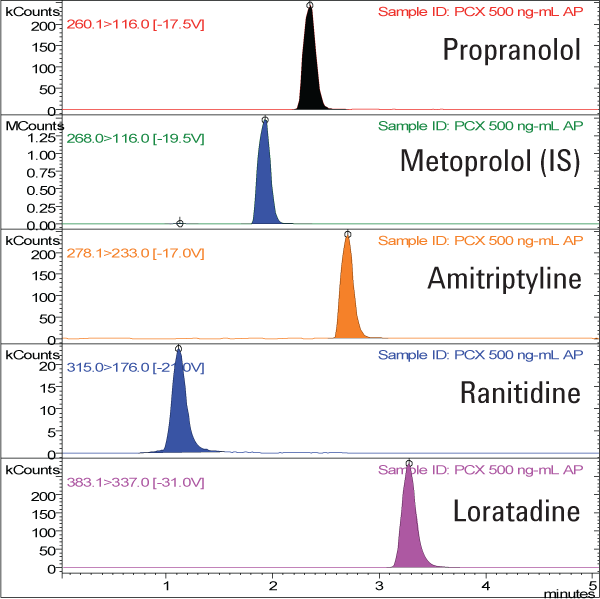
We extracted nonpolar basic compounds in human plasma using Agilent Bond Elut Plexa PCX and separated them on an Agilent Pursuit C18 LC column (Figure 2). The limit of detection of the solid phase extraction and LC/MS/MS analysis was 1.0 ng/mL. Recoveries were excellent, demonstrating good retention and elution, as well as minimal ion suppression. Response for all the compounds evaluated was linear up to three orders of magnitude from 1.0 ng/mL to 1.0 µg/mL, with correlation coefficients all greater than 0.999.
 Enlarge
Enlarge
Figure 2. Chromatograms of a 50 ng/mL drug extract from plasma, extracted using Agilent Bond Elut Plexa PCX and separated on an Agilent Pursuit C18 LC column.
As shown in Table 1, we obtained reproducibly high recoveries with the generic protocol. [2] This protocol is suitable for a wide range of compounds in plasma and other biological samples.
Analyte |
log P |
pKa |
% Recovery1 |
% RSD2 |
% Recovery1 |
% RSD2 |
|---|---|---|---|---|---|---|
Ranitidine |
1.9 |
8.2 |
101 |
5 |
94 |
6 |
Propranolol |
3.6 |
9.5 |
9.5 |
7 |
92 |
4 |
Amitriptyline |
4.6 |
9.4 |
95 |
5 |
91 |
5 |
Loratadine |
5.2 |
9.3 |
100 |
4 |
91 |
4 |
1Recoveries calculated as % of signal intensity of an extracted sample compared to that calibration curve
2RSD = standard deviation/average recovery × 100; n = 6
Table 1. High recoveries of nonpolar basic compounds from human plasma.
Agilent offers a wide range of solutions for forensic/toxicology analysis
Agilent’s range of advanced detection and analysis equipment provides solutions for all forensic/toxicology processes. In addition to a comprehensive range of sample preparation products, LC columns and GC columns, our scanning and measurement solutions include single quadrupole GC/MS systems with unmatched sensitivity; reliable, day-to-day performance of LC/MS analyzers; and advanced automation and workflow management capabilities enabled by MassHunter and OpenLAB software. Contact your Agilent sales representative to explore these options today.
References
- P. J. W. Stone, K. McCann. Comprehensive LC/MS Analysis of Illicit and Pain Management Drugs, Including Their Metabolites, in Urine. Application note, Agilent Technologies, Inc., Publication number 5991-1667EN (2013).
- W. Hudson, A. Junker-Buchheit. Extraction of Non-Polar Basic Drugs from Plasma with Polymeric SPE Cation Exchange, Bond Elut Plexa PCX. Application note, Agilent Technologies, Inc., Publication number SI-01014 (2010).
>> Update My Profile | Subscribe to Access Agilent | Article Directory