Protecting Your Lab's Data
Has Never Been More Important
You see the news reports: Systems being hacked. Data being tampered with and results modified fraudulently.
If you're in a regulated industry—pharma, forensics, energy, chemicals, food safety, environmental testing—you are more concerned than most. There are stringent data-integrity standards that you are required to meet.
Even if your lab isn't highly regulated, you have exacting quality standards of your own—and a need to meet them in the most efficient way possible. Your continued success depends on it.
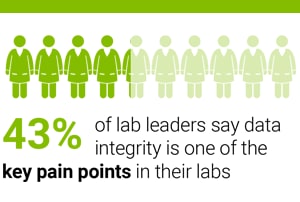
In a seven-country survey of 650 laboratory managers and directors, 43 percent told us that data integrity is one of their key concerns.
That's why we continually evaluate our data systems—across a growing portfolio of analytical systems—to align our design specifications with your evolving needs in a changing world. Technical controls in Agilent OpenLab software, for example, reduce the need for procedural controls.
Imagine: No more shuffling papers and writing in logbooks. All records and signatures are electronic—and the order of approvals is guided and enforced by the system. Even better, the audit trail review is essentially automated, with color-coding to let you see what has already been completed and what remains to be checked. Elegant touches like that let you know that we've done our homework. We understand what you need and what regulators want to see. |
As Bob McDowall, director of R.D. McDowall Limited, put it: "The technical controls available through these electronic systems will significantly improve compliance with data integrity regulations, while also providing the added benefit of efficient, streamlined business processes."
What's more, we stand ready to provide guidance on how to map and manage data-integrity processes and how to address any gaps.
Every business should know the importance of keeping software up to date and have all the latest security measures in place—to benefit from productivity enhancements. Laboratory software is no different, and we regularly update OpenLab to meet your needs.
We also recommend regular visits to our Everyday Data Integrity page, where we post information and insights to help you understand data integrity and management issues and explore your options.
Resources:
- Data Integrity Resource Center
- Does Your Electronic Data System Meet 21 CFR Part 11 and CGMP Requirements?
- Agilent OpenLab Software Suite