 Image courtesy of the Vanderbilt Vaccine Center
Image courtesy of the Vanderbilt Vaccine CenterBefore COVID-19, the idea of pandemic readiness was far from the minds of most people, but pandemic readiness has been a hot topic with researchers for years.
As part of the DARPA - Pandemic Prevention Platform (P3) program, the Vanderbilt Vaccine Center (part of Vanderbilt University Medical Center) has been working towards being able to quickly respond to an epidemic by developing antibody treatments.
In mid-2018, they began investigating both monoclonal antibody development for the treatment and prevention of infectious diseases and the potential of an approach to drastically reduce the time needed to identify and evaluate highly potent antibodies.
Their approach, which relies on bio-informatics and synthetic biology techniques, is also tackling two of the biggest hurdles to large-scale antibody-based treatment: delivery and time. Instead of providing antibodies intravenously, the theory is that messenger RNA encoding the desired antibody protein would be delivered to patients, transforming their cells into antibody factories.
And they're not doing it alone. Collaborations within the greater virology community, including academic institutions such as Washington University in St. Louis, the University of North Carolina at Chapel Hill, and other institutions, ignited conversations about sharing cells as well as technologies, and how to work together to develop an antibody discovery program and get it into the clinic.

The process involves obtaining blood cells from people who have survived COVID-19 and who are now immune. Through a multistep process involving the selective isolation of memory B cells (the antibody-making cells of the immune system) from blood samples, scientists use high-throughput sequencing of the genes encoding for the light and heavy chain pairs of antibodies.
Using a sophisticated bio-informatics approach, the sequences of those antibody light and heavy chain gene pairs, which are most likely to encode for functional antibodies against the virus, are identified and synthesized. These genes are then expressed and manufactured at small scale into antibody proteins, which are tested for virus neutralizing effect. This process can yield hundreds of antibody molecules that need to be individually assessed for virus neutralizing activity. The final step in this process is to make messenger RNA of those antibodies that display virus neutralizing activity and inject it into patients displaying symptoms of the disease. By allowing the patient's own cells to manufacture the antibodies against the virus, this strategy circumvents the need for large-scale manufacturing of antibodies, which can take significant time and face regulatory and logistical hurdles.
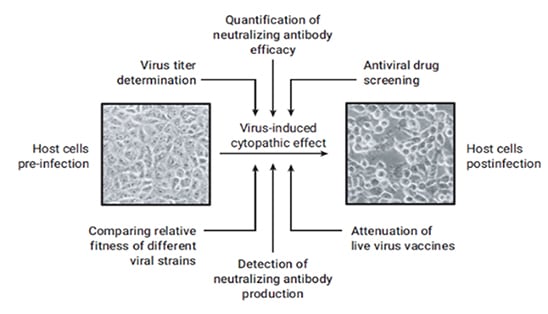
Among other leading-edge technologies being utilized throughout the different stages of the program is the Agilent xCELLigence Real Time Cell Analysis (RTCA) platform, developed by ACEA Biosciences, now a part of Agilent. The accurate measurement of viral activity plays an essential role for viral vaccine development and infectious disease studies. The xCELLigence system is being used to screen the developed antibodies, in a high-throughput and quantitative manner, for their ability to recognize the virus and inhibit the viral infection process in cell culture, referred to as the cytopathic effect (CPE).
Viral CPE in cell culture involves a number of changes in host cell. These changes include cell shrinkage or enlargement, deterioration/lysis, cell fusion, and the formation of inclusion bodies. Not all viruses cause a CPE in their host cell, but if they do, it can be a useful tool for various research applications, including everything from virus titer determination to the detection and quantification of neutralizing antibodies.
The xCELLigence system can monitor CPE in an automated way, precluding the need to use labor-intensive methods such as manually counting the plaques formed by viral infection. Moreover, since the instrument is housed inside a CO2 incubator, it provides for a more secure and safe environment when dealing with highly infectious viruses. This and other analyses can ultimately bring the candidate pool from hundreds of antibodies to just a handful. These antibodies are then are purified and sent to university collaborators to identify the most potent among them.
The ongoing global challenge to contain COVID-19 has reinforced the need for large-scale and timely deployment of safe and efficacious therapies that can provide effective immunity to a broad segment of the world population. An endeavor of this magnitude can only be undertaken through partnerships, cooperation, and communication across governments, industry, and the scientific community, as well as utilization of cutting-edge innovation and technologies.
Virology Research Grant Program
ACEA Biosciences, a part of Agilent, is committed to supporting virology and vaccine research. They are currently accepting xCELLigence research grant applications for virology.
For six months, the research grant winner will be provided:
- xCELLigence Real Time Cell Analysis instrument
- Consumables
- Consultation
Submission must be received by May 29, 2020. Visit the xCELLigence research grant program site to submit your application and learn more.
Supporting Resources:
- Branche, E. et al. Human Polyclonal Antibodies Prevent Lethal Zika Virus Infection in Mice. Sci Rep. 2019 volume 8: 9857
- Charretier C., et al. Robust real-time cell analysis method for determining viral infectious titers during development of a viral vaccine production process. Journal of Virological Methods. 2018 volume 252:57-64
- Pandemic Prevention Platform (P3)