Agilent Seahorse Video Library
Videos explaining how Agilent Seahorse XF Technology works.
Click on an image to view the video in full size.
|
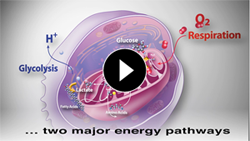
Energy Pathways
What We Measure
Use your Agilent Seahorse XF analyzer to simultaneously measure the two major energy pathways of the cell, Mitochondrial Respiration and Glycolysis, in live cells, in real time.
|

|
Transient Microchamber
How We Measure
Agilent Seahorse XF analyzers utilize a patented transient microchamber and disposable sensor cartridge. No dyes or exogenous labels are required.
|
|
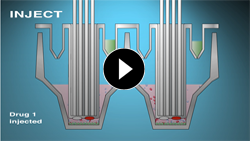
Compound Injection Ports
Kinetic Measurements
Sequentially inject up to four compounds of various types, including inhibitors, stimulators or substrates into each well during the course of an experiment.
|

|
Seeding Cell Culture Microplates
Agilent Seahorse XF96 Analyzer
Whether you are using adherent or suspension cells, primary cells or cell lines, your Seahorse XF data will benefit from following these best practices.
|
Jove Videos

|
Bioenergetics and the Oxidative Burst
Blood leukocytes and platelets can be used as a marker of overall bioenergetic health of an individual and so have the potential to monitor pathological processes and the impact of treatments. Here we describe a method to isolate and measure mitochondrial function and the oxidative burst in these cells.
|

|
Measurement and Analysis of Extracellular Acid Production to Determine Glycolytic Rate
Here, we demonstrate how to gather and correct extracellular flux data to distinguish between respiratory and glycolytic sources of extracellular acidification.
|
|
Metabolic Characterization of Polarized M1 and M2 Bone Marrow-derived Macrophages
Metabolic reprogramming is a characteristic and prerequisite for M1 and M2 macrophage polarization. This manuscript describes an assay for the measurement of fundamental parameters of glycolysis and mitochondrial function in mouse bone marrow-derived macrophages.
|

|
Metabolic Profile Analysis of Zebrafish Embryos
Here we describe a rapid way to measure the in vivo metabolic profile of developing zebrafish that allows the comparison of different mitochondrial function parameters between genetically or pharmacologically manipulated embryos, thereby increasing the applicability of this organism.
|

|
Preparation and Respirometric Assessment of Mitochondria from Skeletal Muscle Tissue
Methods for biopsy of Vastus lateralis, preparation of purified mitochondria, and respirometric profiling are described. The use of small muscle volume makes this technique suitable for clinical research applications.
|

|
Profiling Mitochondrial Function in C2C12 Myoblast Cells
A description of a method for profiling mitochondrial function in cells is provided. The mitochondrial profile generated provides four parameters of mitochondrial function that can be measured in one experiment: basal respiration rate, ATP-linked respiration, proton leak, and reserve capacity.
|
|
Real Time Analysis of Metabolic Profile in Ex Vivo Mouse Intestinal Crypt Organoid Cultures
Small intestinal crypt organoids cultured ex vivo provide a tissue culture system that recapitulates growth of crypts dependent on stem cells and their niche. We established a method to assay the metabolic profile in real time in primary mouse crypt organoids.
|

|
Pancreatic Cancer Stem Cell Characteristics Developing New Treatment Strategies
Pancreatic cancer stem cells (CSCs) can be expanded in vitro using the anchorage-independent sphere culture technique, which represents a powerful tool to study CSC biology and can serve as the first step to develop novel CSC-targeting therapies.
|

|
High Throughput Microplate Respiratory Measurements
Step-by-step instructions for the performance of a collection of microplate based respirometric assays using isolated mitochondria from minimal quantities of mouse skeletal muscle.
|
For Research Use Only. Not for use in diagnostic procedures.












